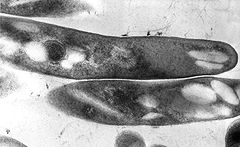
Mycobacteria
| Mycobacterium | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
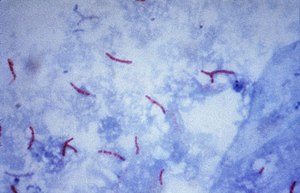
Mycobacterium tuberculosis ( Ziehl-Neelsen stain ) |
||||||||||
| Systematics | ||||||||||
|
||||||||||
| Scientific name | ||||||||||
| Mycobacterium | ||||||||||
| Lehmann & Neumann 1896 |
The mycobacteria ( Mycobacterium ) form a genus consisting of approx. 100 species. They are the only members of the Mycobacteriaceae family . As triggers of mycobacteriosis , they include human pathogens such as Mycobacterium tuberculosis ( tuberculosis ) and Mycobacterium leprae ( leprosy ), pathogens in animals such as the causative agent of bovine tuberculosis ( Mycobacterium bovis ), but also free-living species.
Mycobacteria are difficult to stain with the Gram stain, but their cell wall structure largely corresponds to the wall structure of Gram-positive bacteria, i.e. H. the cell wall has no outer membrane and consists of a multilayered peptidoglycan (murein). The classification as a gram-positive bacteria was also confirmed by RNA analyzes. Due to the high GC content in their DNA, they are classified as Actinobacteria , a division of gram-positive bacteria.
features
general characteristics
Mycobacteria are always dependent on oxygen (obligatory aerobic ) and need organic substances for energy gain ( chemoorganotroph ). They are usually rod-shaped and rarely form branches in older cultures, which then often disintegrate again into cocci (spheroidal bacteria) or rods. In contrast to this, the majority of actinomycetes form branched mycelia, which resemble the mycelia formed by fungi ; one speaks of filamentous actinomycetes. The name is based on this property, actinomycetes means something like “ray mushrooms” (Greek “aktis” for ray and “mykes” for mushroom).
Cell envelope
One of the main characteristics of mycobacteria is that a large part of the cell wall components act as antigen components . They provoke an immune reaction in host organisms and lead to a late-type allergy ( type IV ). The best known reaction is the tuberculin reaction .
Characteristic are the high lipid content of the cell wall, the mycolic acids, the phthiocerol outer shell and especially in Mycobacterium tuberculosis and Mycobacterium bovis the cord factor , the compounds of mycolic acids with the disaccharide trehalose . The cord factor leads to the cord-like or plait-like growth in older cultures.
The very high acid resistance is due to the long-chain mycolic acids . An arabinogalactan complex is bound to every 10th N-acetylmuramic acid (which belongs to the backbone of the peptidoglycan ). This consists of a linear galactose strand and is branched with arabinose chains. Mycolic acids are in turn bound to these complexes. Finally, the DIM / DIP layer is not covalently attached to the outward-pointing mycolic acids .
The cell wall of the mycobacteria therefore has something like another outer bilayer .
Because of this cell wall structure, mycobacteria have a very high resistance. This means that the obligate parasitic mycobacteria remain infectious for several months under favorable conditions in the wild and, with a few exceptions (e.g. streptomycin or kanamycin), are resistant to most antibiotics . They are also more resistant than other bacteria to most alkalis and acids. The acid resistance can be demonstrated by the Ziehl-Neelsen staining . After staining with aniline dyes (e.g. carbol fuchsin), the bacterial cells can no longer be discolored with acid, an effect that Paul Ehrlich first noticed in collaboration with Robert Koch in 1882 . Acid resistance only occurs in a few types of bacteria, such as Nocardia , Rhodococcus and Corynebacterium .
physiology
Mycobacteria use u. a. Triacylglycerine (neutral fats, TAGs) as reserve substances. These neutral fats are accumulated in granules, so-called "fat bodies", within the cell body. Triacylglycerols as storage lipids are widespread in eukaryotes such as yeasts and fungi, but they are less common among bacteria. The intracellular accumulation of TAGs has been demonstrated especially in the Actinomycetales, such as Mycobacterium , Streptomyces , Rhodococcus and Nocardia . Certain waxes, so-called wax esters , are also used by some representatives of the actinomycetals as storage substances. In the mycobacteria they were z. B. found in Mycobacterium tuberculosis . Wax esters as reserve substances occur rather rarely in the bacterial kingdom, except for the Actinomycetales they were z. B. still in Moraxella and Alcanivorax , both belonging to the proteobacteria , detected. Wax esters as reserve substances are rarely found in eukaryotes, e.g. B. in seeds of the jojoba plant. Also glycogen and trehalose be considered as a possible storage materials of mycobacteria.
The citric acid cycle is changed in various mycobacteria (including Mycobacterium tuberculosis ) : the E1 subunit of ketoglutarate dehydrogenase is replaced by a ketoglutarate decarboxylase , which initially produces succinate semialdehyde independently of coenzyme A , which is derived from an NADP + -dependent succinate- Semialdehyde dehydrogenase is dehydrated to succinate .
Occurrence
Only a few of the 100 or so species are parasites and depend on the host as a habitat. In contrast to the Mycobacterium tuberculosis complex, which is mostly dependent on the hosts, the majority of species live freely in the environment and are not pathogenic (non-pathogenic). These species are classified as non-tuberculous, non-pathogenic mycobacteria. It is believed that most of the species are saprophytic ; H. lives from the decomposition of dead organic matter. They have been found in soil and groundwater, dust, and freshwater and seawater.
Free living mycobacteria
Examples of mycobacteria occurring in the soil are the Mycobacterium terrae complex ( M. terrae , M. triviale and M. nonchromogenicum ) and M. fortuitum . The latter was also isolated from seawater; other species found in the sea are Mycobacterium chelonae , M. marinum and M. gordonae . Some species are found in man-made environments such as sewage, sewage sludge, or drinking water. Species such as Mycobacterium gordonae , M. chelonae subsp. chelonae and M. flavescens detected. The species M. gordonae , also known in English under the name "tap water bacillus", is probably the most common mycobacterium in drinking water. Some species, such as M. kansasii and M. avium , are even able to reproduce in treated (distilled) drinking water and are difficult to remove from infected tap water systems, which makes them a significant hazard in hospitals. Similar species compositions were found in wastewater as in soils and freshwater. As mentioned above, the obligate pathogenic species of the Mycobacterium tuberculosis complex that only show growth in the host cells can survive outside the host for a while. Mycobacterium bovis is said to be able to grow for up to 13 days in the faeces of cattle .
Mycobacterium tuberculosis complex
Most representatives of the obligately pathogenic Mycobacterium tuberculosis complex (such as Mycobacterium tuberculosis , Mycobacterium bovis , Mycobacterium africanum or Mycobacterium microti ) live as obligate intracellular parasites in macrophages . Virulence factors are not known, but their special wall structure, which contains waxy substances and mycolic acids , protects against external influences. The lipids of the cell wall are also the cause of the characteristic acid resistance. The wall structure prevents a rapid exchange of substances with the environment and thus causes only slow growth and reproduction. This extremely slow growth in comparison to other bacteria is characteristic of all mycobacteria.
Furthermore, although no life cycle as a soil bacterium has been proven for the members of the Mtb complex, they have not lost their ability to survive in amoebas or cysts attached to them. Rather, it is this property of the mycobacteria in general that made it easier for the Mtb-complex members to survive asleep in the (amoeba-like) phagocytes of their hosts for a long time without cell division (latent infection).
Growth rate
With regard to the growth rate, the mycobacteria are divided into two groups: the slow-growing ("slow growers") with a generation time of 6–24 hours in laboratory cultures and the fast-growing ("rapid growers") with a generation time of 1–4 hours. For comparison: the generation time of Escherichia coli in laboratory cultures is 20 minutes under favorable conditions. Fast-growing mycobacteria form macroscopically visible colonies within a week, slow-growing mycobacteria take up to 8 weeks for this. Most of the (obligatory or facultative) pathogens can be found among the slowly growing mycobacteria, many of the “rapid growers” are non-pathogenic. The subdivision into slow and fast growing mycobacteria is also phylogenetically relevant, it reflects evolutionary relationships.
Systematics and subdivision
The mycobacteria belong to the order Actinomycetales . According to their shape, mycobacteria are classified between the corynebacteria and the proactinomycetes (actinomycetes that do not form a permanent mycelium such as Nocardia ), there are also close relationships with Rhodococcus and Caseobacter (now placed with Corynebacterium ).
The genus is taxonomically divided into three groups:
- The Mycobacterium tuberculosis complex, the causative agents of tuberculosis (including Mycobacterium tuberculosis , M. bovis , M. microti and M. africanum )
- Mycobacterium leprae , the causative agent of leprosy
- All other species are classified as non-tubercular mycobacteria , also known as MOTT (English: mycobacteria other than tuberculosis). These are free-living bacteria that rarely cause disease (facultative pathogenic ).
Subdivision according to Runyon
According to Runyon , the mycobacteria are subdivided according to growth rate and pigment formation during exposure (so-called photochromogenicity ). The pigment behavior, however, is not phylogenetically relevant.
- Group I: Photochromogenic, slow growers mycobacteria: They only form yellow color pigments under the influence of light. Example species: Mycobacterium kansasii , M. marinum , M. asiaticum and M. simiae .
- Group II: Skotochromogenic slow growers produce pigments even in the dark. Examples: Mycobacterium scrofulaceum , M. szulgai and M. xenopi .
- Group III: Non-chromogenic slow growers never form pigments. Example species: Mycobacterium ulcerans , the causative agent of Buruli ulcer and the Mycobacterium avium complex (MAC), consisting of Mycobacterium intracellulare and Mycobacterium avium .
- Group IV: Fast-growing mycobacteria that form colonies that are clearly visible on agar media within a week, e.g. B. Mycobacterium fortuitum .
The entire Mycobacterium tuberculosis complex and Mycobacterium leprae belong to group III. Apathogenic, non-pathogenic mycobacteria such as M. moriokaense mainly come from the group of fast-growing, non-tubercular mycobacteria (group IV).
species
The Mycobacterium tuberculosis complex:
- Mycobacterium africanum Castets et al. 1969
- Mycobacterium canettii
- Mycobacterium caprae
- Mycobacterium bovis Karlson & Lessel 1970 , causative agent of tuberculosis in cattle
- Mycobacterium microti Reed 1957
- Mycobacterium mungi discovered in 2010 , infects zebra mongoose
- Mycobacterium pinnipedii Cousins et al. 2003
- Mycobacterium tuberculosis (Zopf 1883) Lehmann & Neumann 1896 , causative agent of tuberculosis
Some non-tuberculous mycobacteria (NTM):
- Mycobacterium genavense ; rarely causes lymphadenitis and dissiminated infections
- Mycobacterium immunogenicum ; rarely causes skin diseases
- Mycobacterium conspicuum ; rarely cause of severe dissimilar infections
- Mycobacterium mucogenicum ; rarely cause of severe dissimilar infections
- Mycobacterium ulcerans ; Buruli ulcer pathogen
- Mycobacterium xenopi Schwabacher 1959
- Mycobacterium shottsii Rhodes et al. 2003
-
Mycobacterium avium complex
- Mycobacterium avium Chester 1901 , causative agent of avian tuberculosis
- Mycobacterium avium subsp. paratuberculosis (Bergey et al. 1923) Thorel et al. 1990 (synonym: Mycobacterium paratuberculosis Bergey et al. 1923 ), causative agent of chronic enteritis in cattle
- Mycobacterium intracellulare (Cuttino and McCabe 1949) Runyon 1965
- Mycobacterium smegmatis (Trevisan 1889) Lehmann & Neumann 1899
- Mycobacterium kansasii Hauduroy 1955
-
Mycobacterium terrae complex: The species in this complex belong to group III (slow growing, unpigmented) and are associated with lung diseases.
- Mycobacterium nonchromogenicum Tsukamura 1965
- Mycobacterium terrae Wayne 1966
- Mycobacterium triviale Kubica 1970
The following types are considered non-pathogenic, i.e. H. until now they have not been associated with human diseases. All the species listed here belong to the fast-growing mycobacteria (group IV):
- Mycobacterium brumae Luquin et al. 1993
- Mycobacterium chitae Tsukamura 1967
- Mycobacterium confluentis Kirschner et al. 1992
- Mycobacterium fallax Lévy-Frébault et al. 1983
- Mycobacterium moriokaense Tsukamura et al. 1986
Mycobacteria as pathogens
Some of the bacteria that normally live in the wild can cause disease in people with compromised immune systems (pathogenic nontuberculous mycobacteria). Some are also dangerous pathogens in animals and can cause major problems in animal husbandry or agriculture.
Many of the around 100 described species are non-pathogenic to humans or only very rarely cause diseases. Others are facultative pathogenic; they are only dangerous to humans under certain circumstances, for example when the immune system is weakened. None of the pathogenic species (except for M. tuberculosis ) are transmitted from person to person. Many species occur in soils and are saprophytic . Mycobacteria , which rarely cause disease, include Mycobacterium triviale , M. gordonae and M. nonchromogenicum .
In agriculture and animal husbandry, Mycobacterium bovis has the greatest economic importance among the mycobacteria. Other important species in agriculture are Mycobacterium avium , M. paratuberculosis (or Mycobacterium avium subsp. Paratuberculosis ) and Mycobacterium tuberculosis . Mycobacterium avium can cause avian tuberculosis in turkeys, chickens, pigeons and other birds; the bacterium also rarely occurs in pigs, horses and cattle. Also, M. tuberculosis can cause tuberculosis in dogs, cats, pigs and cattle. M. paratuberculosis , now listed as a subspecies of Mycobacterium avium , is the causative agent of paratuberculosis (John's disease) in cattle. M. paratuberculosis is harmless to humans , while M. avium and M. intracellulare can also be pathogenic for humans and cause lung diseases, for example. Mycobacterium avium and Mycobacterium avium ssp. paratuberculosis form the “ Mycobacterium avium complex” (MAC) with M. intracellulare .
Cellular Routes of Infection
There are currently two ways in which mycobacteria can infect cells: lytic and non-lytic infection of cells.
In lytic infection, the bacteria are absorbed by phagocytes into the endosomes in order to survive or multiply there. In the latter case they get into the cytosol and induce the lysis of the host cells, which perishes in the process. The released mycobacteria can then infect other cells.
In addition to the well-known course of lytic infection, a further route of infection, non-lytic infection or non-lytic discharge, was discovered in M. tuberculosis and M. marinum in 2009 . So-called ejectosomes are used, regions of the membrane enriched with actin filaments: these filament complexes allow the bacteria to leave the host cell without causing a lethal leak in the membrane.
In the lytic route of infection, the bacteria are released and can thus be successfully fought with antibiotics . On the other hand, the medical importance of non-lytic infection lies in the fact that the bacteria can spread from cell to cell in the tissue without being bothered by the currently available drugs .
Mycobacterium tuberculosis complex
The Mycobacterium tuberculosis (Mtu) complex created on the basis of rRNA analyzes includes Mycobacterium tuberculosis , M. africanum , M. bovis , M. microti and M. canettii . In recent years, the species M. caprae (previously listed as the subspecies Mycobacterium tuberculosis subsp. Caprae ) and M. pinnipedii have been added to this group. The vaccine strain Bacillus Calmette-Guérin (BCG) produced by M. bovis is also part of the complex.
Based on the investigation of VNTRs , it is assumed that the tribes of the Mtu complex evolved from the ancestor of the Mtu complex at the earliest about 40,000 years ago and were distributed with humans in the different regions. It can also be concluded from this that the strains that infect the animals developed from strains that infected humans, i.e. that the transmission originated from humans.
Pathogenicity of the species of the Mycobacterium tuberculosis complex
Mycobacterium tuberculosis is transmitted through droplet infection . Mycobacterium tuberculosis can also be transmitted from humans to animals. In humans, in addition to the diseases caused by Mycobacterium tuberculosis , only those caused by M. bovis and M. africanum are of significant frequency; the other representatives of the Mycobacterium tuberculosis complex only very rarely cause tuberculosis in humans.
Humans are the primary main hosts in the complex only for Mycobacterium tuberculosis , M. africanum and M. canettii . The other types of bacteria in the complex are primarily pathogenic for animals, but can also be transmitted to humans and, especially if there is an immunodeficiency, have a human pathogenic effect and trigger tuberculosis. These infections are therefore zoonoses , and secondary transmission from humans to animals is also possible. Mycobacterium africanum is a common tuberculosis pathogen in Africa, but this species may be a variant of Mycobacterium tuberculosis . Mycobacterium bovis is a parasite that occurs in cattle and causes bovine tuberculosis , but it can be transmitted to humans, e.g. B. by ingesting unpasteurized milk. Mycobacterium microti is the cause of tuberculosis in voles and can be transmitted from here to humans as a tuberculosis pathogen. Mycobacterium pinnepedii is pathogenic for seals (seal tuberculosis) and Mycobacterium caprae for goats; transmission to humans is rare.
Non-tuberculous mycobacteria
The non-tuberculous mycobacteria (NTM) only rarely cause disease (mostly in people with a severely weakened immune system); they are considered to be facultative pathogenic. Some of them have not yet been proven to cause illnesses; they are considered non-pathogenic.
The non-tuberculous mycobacteria did not receive attention in medicine until a long time after the description of Mycobacterium tuberculosis and M. leprae . Above all, the increased occurrence of the immunodeficiency disease AIDS in recent years has led to an increase in diseases caused by non-tuberculous mycobacteria. In the case of infections, these types mostly produce non-tuberculous pneumonia, skin diseases (e.g. Buruli ulcer caused by the M. ulcerans species that occurs in the tropics and Australia ) and involvement of the lymph nodes.
Other names for this group include Mycobacteria other than tuberculosis (MOTT) and “atypical mycobacteria”. Since they occur freely in the environment, they are also referred to as "potentially pathogenic environmental (environmental) mycobacteria" (PPUM or PPEM).
Pathogenic nontuberculous mycobacteria
Direct transmission from person to person is usually not possible. The infection usually occurs via infected materials or airborne (droplet infection). The non-tuberculous mycobacteria are often insensitive to drugs that are used to treat tuberculosis ( antituberculotics ) and are generally highly resistant to external influences.
Some pathogenic species of group I: Mycobacterium kansasii can cause non-tuberculous lung diseases, human infections of Mycobacterium marinum can e.g. B. occur in swimming pools and cause granulomas (swimming pool granulomas ). Mycobacterium scrofulaceum , the worldwide widespread pathogen of lymphadenitis, is one of the pathogenic species of group II .
Group III species are Mycobacterium malmoense (can cause lung diseases), Mycobacterium avium (trigger of avian tuberculosis in chickens, turkeys, pigeons and other birds, rarely it also infects pigs, cattle or horses). In humans, it can cause lung disease and (in children) lymphadenitis . Mycobacterium avium is the most common pathogenic mycobacterium in AIDS patients and, together with Mycobacterium intracellulare , is assigned to the Mycobacterium avium complex (MAC) established on the basis of genetic studies . In the case of the Mycobacterium avium-intracellulare-scrofulaceum complex (MAIS), the species Mycobacterium scrofulaceum is also added. M. ulcerans causes Buruli ulcer , a skin disease that occurs in the tropics.
Group IV pathogenic species are Mycobacterium chelonae , which causes skin or soft tissue diseases, abscesses and bone or joint diseases. Like Mycobacterium fortuitum , also belonging to group IV, it has a high resistance to disinfectant solutions and is therefore a possible risk of infection in the clinic when using implants.
literature
- Marianne Abele-Horn: Antimicrobial Therapy. Decision support for the treatment and prophylaxis of infectious diseases. With the collaboration of Werner Heinz, Hartwig Klinker, Johann Schurz and August Stich, 2nd, revised and expanded edition. Peter Wiehl, Marburg 2009, ISBN 978-3-927219-14-4 , pp. 238-251.
- Karl Bernhard Lehmann, Rudolf Otto Neumann: Atlas and outline of bacteriology and textbook of special bacteriological diagnostics . Lehmann, Munich 1896.
- R. Schulze-Roebbecke: Mycobacteria in the environment . In: Immunity and Infection . Volume 21, Issue 5, 1993
- Martin Dworkin, Stanley Falkow, Eugene Rosenberg, Karl-Heinz Schleifer, Erko Stackebrandt (eds.): The Prokaryotes , 3rd edition, Vol. 3: Archaea. Bacteria: Firmicutes, Actinomycetes . Springer Verlag, New York 2006, ISBN 978-0-387-25493-7 (print), ISBN 978-0-387-30743-5 (online)
- Juan Carlos Palomino, Sylvia Cardoso Leão, Viviana Ritacco: Tuberculosis 2007 . On-line
- D. Schlossberg: Tuberculosis & Nontuberculous Mycobacterial Infections . 5th edition, McGraw-Hill Publishing Company, 2006 ISBN 0-07-143913-7
- Hett EC, Rubin EJ: Bacterial growth and cell division: a mycobacterial perspective . In: Microbiol. Mol. Biol. Rev. . 72, No. 1, March 2008, pp. 126-56, table of contents. doi : 10.1128 / MMBR.00028-07 . PMID 18322037 . PMC 2268284 (free full text).
Web links
- Triglycerides and wax esters in bacteria - Westfälische Wilhelms-Universität Münster, IMMB - Working group Prof. Dr. Alexander Steinbüchel
- JP Euzéby: List of Prokaryotic Names with Standing in Nomenclature - Genus Mycobacterium
Individual evidence
- ↑ Brennan PJ, Nikaido H: The envelope of mycobacteria . In: Annu. Rev. Biochem. . 64, 1995, pp. 29-63. doi : 10.1146 / annurev.bi.64.070195.000333 . PMID 7574484 .
- ↑ a b Michael Rolle, Anton Mayr (ed.): Medical microbiology, infection and epidemic theory. 7th edition. Enke Verlag, Stuttgart 1993, ISBN 3-432-84686-X
- ^ PJ Brennan: Mycobacterium and other actinomycetes . In: C. Ratledge, SG Wilkinson (Ed.): Microbial Lipids . Academic Press, London 1988, Vol. 1, pp. 203-298.
- ↑ a b c d Sybe Hartmans, Jan de Bont, Erko Stackebrandt: The Genus Mycobacterium nonmedical In: The Prokaryotes, A Handbook of the Biology of Bacteria . 7 volumes, 3rd edition, Springer-Verlag, New York et al. O. 2006, Volume 3: Archaea. Bacteria: Firmicutes, Actinomycetes. ISBN 0-387-25493-5
- ↑ a b c Jessup Shively: Inclusions in Prokaryotes , Springer-Verlag, 2006, ISBN 978-3-540-26205-3
- ↑ Tian J, Bryk R, Itoh M, Suematsu M, Nathan C: Variant tricarboxylic acid cycle in Mycobacterium tuberculosis: identification of alpha-ketoglutarate decarboxylase . In: Proc. Natl. Acad. Sci. USA . 102, No. 30, July 2005, pp. 10670-5. doi : 10.1073 / pnas.0501605102 . PMID 16027371 . PMC 1180764 (free full text).
- ↑ a b c d R. Schulze-Roebbecke (1993)
- ^ F. Mba Medie, I. Ben Salah a. a .: Mycobacterium tuberculosis complex mycobacteria as amoeba-resistant organisms. In: PLOS ONE . Volume 6, number 6, 2011, p. E20499. doi : 10.1371 / journal.pone.0020499 . PMID 21673985 . PMC 3108610 (free full text).
- ↑ a b c D. Schlossberg (2006)
- ↑ Alexander KA, Laver PN, Michel AL, et al. : Novel Mycobacterium tuberculosis complex pathogen, M. mungi . In: Emerging Infect. Dis. . 16, No. 8, August 2010, pp. 1296-9. PMID 20678329 .
- ↑ Marianne Abele-Horn (2009), p. 246.
- ↑ Marianne Abele-Horn (2009), p. 246.
- ↑ Hagedorn, M. et al .: Infection by tubercular mycobacteria is spread by nonlytic ejection from their amoeba hosts . In: Science . 323, No. 5922, 2009, pp. 1729-1733. PMID 19325115 .
- ↑ a b Mario C. Raviglione (ed.): Reichman and Hershfield's tuberculosis: A comprehensive international approach , Part B, Informa Healthcare, New York [ua] 2006, ISBN 0-8493-9271-3
- ↑ Wirth T, Hildebrand F, Allix-Béguec C, et al. : Origin, spread and demography of the Mycobacterium tuberculosis complex . In: PLoS Pathog . . 4, No. 9, 2008, p. E1000160. doi : 10.1371 / journal.ppat.1000160 . PMID 18802459 . PMC 2528947 (free full text).
- ^ BC de Jong, M. Antonio, S. Gagneux: Mycobacterium africanum - review of an important cause of human tuberculosis in West Africa. In: PLoS neglected tropical diseases. Volume 4, number 9, 2010, p. E744, doi : 10.1371 / journal.pntd.0000744 . PMID 20927191 . PMC 2946903 (free full text). (Review).
- ↑ a b Juan Carlos Palomino, Sylvia Cardoso Leão and Viviana Ritacco (2007) Online ( Memento of the original from July 17, 2011 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.