Selenoketones
| Selenoketones |
|---|

|
| The selenocarbonyl group is marked in blue . The radicals R 1 and R 2 are organic radicals ( alkyl , aryl or the like). |
Selenoketones (Selone) are organic , chemical compounds . They represent the selenium analogues of ketones and belong to the selenocarbonyl compounds .
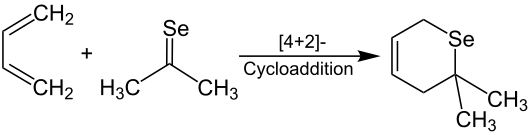
Their functional group consists of a carbon and a selenium atom , which are linked by a double bond . The radicals R 1 and R 2 on the carbon atom of the selenocarbonyl group are organic radicals ( alkyl radical , aryl radical, etc.). Selenoketones with the same radicals (R 1 = R 2 ) are symmetrical selenoketones, while selenoketones with unequal radicals (R 1 ≠ R 2 ) are referred to as asymmetrical selenium ketones. Analogous to the other carbonyl compounds of the chalcogen group (e.g. thiocarbonyl compounds ), it is a reactive class of substances. Several methods for producing stable selenoketones are known today. They can be used as chiral derivatization reagents for the stereoselective control of aldol reactions . They are also suitable for introducing selenium atoms into heterocyclic compounds. Both simple and sterically demanding selenoketones can occur as dimers . Selenoketones are known to enter into cycloadditions with 1,3- dienes in analogy to Diels-Alder reactions .
synthesis
Selenoketones can be synthesized from ketones such as acetone by reacting them with hydrogen selenide under acid catalysis . This creates selenoacetone:
Since hydrogen selenide is extremely toxic , further syntheses have been developed. For example, phosphor ylides can be reacted with elemental selenium . So you can z. B. synthesize selenobenzophenone. Alternatively, hydrazones of the phosphorus ylides can be pyrolyzed with selenium . This variant enables many synthesis variants of stable, sterically strongly hindered selenoketones in good yields. Hydrazones can also be reacted with selenium (I) chloride or bromide and triethylamine to form selenoketones. The action of bis (dimethylaluminum) selenide on ketones is also described as a suitable method. Selenoketones can also be synthesized by the [3,3] sigmatropic rearrangement of allyl alkene selenides .
Reactions
Although the synthesis of stable, monomeric selenoketones is possible, the products are often obtained as dimers . Two monomers of selenoacetone dimerize z. B. to the dimer diselenoacetone.
This is isolated as a clear, red oil and smells like garlic . The dimerization is often reversible . The monomer of selenobenzophenone is stable, but can be converted into the dimer . Unstable monomers of the selenoketones can be stabilized vinylogically ( vinylogy principle ), by sterically demanding residues (e.g. tert - butyl groups ) or as ligands in complexes with transition metals (e.g. nickel , platinum ). The formation of the complex reduces the reactivity of the selenoketones and thus increases their manageability. Furthermore, selenoketones are considered to be good dienophiles in Diels-Alder reactions with 1,3- dienes . This procedure is well suited for introducing selenium into heterocycles . The selenopyran is obtained selectively by reacting selenoacetone with 1,3-butadiene .
In addition, selenoketones can be reduced to selenols with sodium borohydride . The subsequent oxidation in atmospheric oxygen leads to diselenides .
See also
Individual evidence
- ^ A b Paulmier, C. (1986): Selenium Reagents and Intermediates in Organic Synthesis . 4th volume. Oxford: Pergamon Books. Pp. 60-63, ISBN 0-08-032484-3 .
- ↑ Silks, L. et al .: Chiral N-Acetyl Selone-Promoted Aldol Reactions . Synthetic Communications , 2009 , 39 (4) , pp. 641-653. doi: 110.1080 / 00397910802419706 .
- ↑ a b c Schönberg, A. & Wagner, A. (1955): Methods for the production and conversion of thioaldehydes and thioketones. In Müller, E. (Ed.): Methods of Organic Chemistry . Volume IX: sulfur, selenium, tellurium compounds. Stuttgart: Thieme Verlag. Pp. 1199-1202.
- ↑ a b c Erker, G. eta al .: Synthesis and Cycloadditions of Monomeric Selenobenzophenone . Angewandte Chemie International Edition , 1990 , 29 (9) , p. 1067. doi: 10.1002 / anie.199010671 .
- ↑ a b Guzirc, FS (1987): The Chemistry of Selenocarbonyl Compounds. In Liotta, D. (Ed.): Organoselenium Chemistry . New York: Wiley. Pp. 277-324, ISBN 0-471-88867-2 .
- ↑ Ishii, A., Okazaki, R. & Inamoto, N .: Synthesis and reactions of selenoketones . Bulletin of the Chemical Society of Japan , 1988 , 61 (3) , pp. 861-867. doi: 10.1246 / bcsj.61.861 .
- ↑ Segi, M. eta al .: Novel route to selenoketones from ketones by the use of bis (dimethylaluminum) selenide . Tetrahedron , 1989 , 30 (16) , pp. 2095-2098. doi: 10.1016 / S0040-4039 (01) 93721-9 .
- ↑ Shimada, K. eta al .: Generation of a Selenoaldehyde, a Selenoketone, and Telluroaldehydes by [3, 3] Sigmatropic Rearrangement of Allyl Alkenyl Selenides and Tellurides . Chemistry letters, 1995 , 24 (2) , pp. 135-136. doi: 10.1246 / cl.1995.135 .
- ↑ Shigetomi, T. et al .: Synthesis of Selenoketone – Platinum Complex . Bulletin of the Chemical Society of Japan , 2007 , 80 (2) , pp. 395-399. doi: 10.1246 / bcsj.80.395 .
- ↑ Okazaki, R. & Tokitoh, N .: Heavy ketones, the heavier element congeners of a ketone . Accounts of Chemical Research , 2000 , 33 (9) , pp. 625-630. doi: 10.1021 / ar980073b. PMID 10995200 .
- ↑ Fischer, H. et al .: [4+ 2] cycloadditions with transition metal coordinated selenoaldehydes and selenoketones as heterodienophiles . Angewandte Chemie , 1986 , 98 (1) , pp. 80-81. doi: 10.1002 / anie.19860980109 .