Trichoplax adhaerens
| Trichoplax adhaerens | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
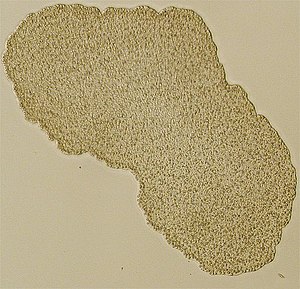
Light microscopic picture of Trichoplax |
||||||||||
| Systematics | ||||||||||
|
||||||||||
| Scientific name of the tribe | ||||||||||
| Placozoa | ||||||||||
| Grell , 1971 | ||||||||||
| Scientific name of the genus | ||||||||||
| Trichoplax | ||||||||||
| FE Schulze , 1883 | ||||||||||
| Scientific name of the species | ||||||||||
| Trichoplax adhaerens | ||||||||||
| FE Schulze , 1883 |
Trichoplax adhaerens is the only generally recognized species of Placozoa. The organism was discovered in 1883 by the German zoologist Franz Eilhard Schulze in a seawater aquarium at the Zoological Institute in Graz . The generic name given by him to the animal is derived from the ancient Greek θρίξ thrix "hair" and πλάξ plax "plate", the Latin epithet adhaerens means "adhesive", so that the species name can be translated as "adhesive hairy plate".
The Placozoa or plate animals are structurally the simplest of all multicellular animals (Metazoa) and form their own animal phyla . The scientific name literally means "flat animals", in German the name disc animals is occasionally used. Based on genetic data, it is certain that the Placozoa include numerous species. Since they morphologically but can not be distinguished, has only been trichoplax the only species described .
For a long time, the animals that had not been observed in their natural habitat were considered to be juvenile stages of cnidarians (Cnidaria): For more than half a century they were interpreted as planula larvae of the hydrozoan species Eleutheria krohni . Only works from the 1970s, including by the Tübingen protozoologist Karl G. Grell , clarified the early phases of the embryonic development of the animals and made a significant contribution to the establishment of their own animal strain.
construction
Placozoa, as their name suggests, usually have a strongly flattened, disc-shaped body. In most cases it is less than half a millimeter in diameter. Occasionally specimens reach sizes of 2 to 3 millimeters - rarely more. The slice is usually only about 25 micrometers thick. With the naked eye, the grayish colored animals, which are transparent in the backlight, can usually just barely be seen. On the surface, they look like large amoebas and, like these, are constantly changing their external shape. In addition, spherical life stages rarely occur, which may be used for passive transport into new habitats.
As the assignment to the tissue- less already indicates, Trichoplax adhaerens tissue and organs are missing ; there is also no pronounced body symmetry, so that neither front nor back nor left or right can be distinguished.
Cells and cell clusters
Both structurally and functionally, Trichoplax adhaerens can be distinguished between a back or dorsal side and an abdominal or ventral side. Both consist of a single layer, to the outside covered with mucus cell layer and remember mainly due to the differences in cell connections, the Gürteldesmosomen to epithelial tissue . In contrast to a real epithelium, however, the placozoa cell layers have no basal lamina ; This means a thin layer made of extracellular material, underlying the epithelium from the inside, which stiffens it and separates it from the interior of the body. The lack of this structure, which is otherwise found in all animals except for a few sponges, can be explained functionally: A stiff separating layer would make the amoeboid changes in shape of Trichoplax adhaerens impossible. To emphasize the difference, you sometimes speak instead of an epithelium, therefore, in the Placozoa by an epithelioid .
A fully grown individual consists of up to a thousand cells that can be assigned to six different cell types (only four were previously known). The simply flagellated cells of the back epithelium are flattened on the outside and form a plate-like cell structure from which the cell bodies protrude into the interior, they contain small lipid bodies , possibly defense substances against predators. Between them there are a few cells with a cup-shaped nucleus that contain a crystalline inclusion and are therefore called crystal cells; they could have a function as sensory cells. The cells on the abdomen are also simply flagellated, they have an elongated columnar shape with a small cross-section on the surface, so that the flagella are very close together on the abdomen and form a ciliated “creep sole”. Their numerous microvilli suggest that they are also involved in the digestion of prey. In between, especially at the edge on the ventral side, there are also cilium-lashed gland cells that can synthesize neuropeptides . These could be involved in the control and regulation of the animal's movement. A third type of non-ciliated cells, the so-called lipophilic cells, extend with their cell body far into the interior of the organism; they are most common in the middle of the crawl sole, i.e. exactly opposite to the distribution of the gland cells. Presumably these cells synthesize digestive enzymes.
Between the two cell layers there is a liquid-filled interior in which there is a loose network of fiber cells, predominantly arranged in one layer. These consist of the cell body with six or more long processes that branch out finely at the end. Contrary to earlier assumptions, the fiber cells probably do not form a syncytium . The processes pull themselves to the cells of both epithelia and to other fiber cells, they connect all somatic cells to a network. The contact points are morphologically unspecialized, they do not form synapses or similar formations. Partitions (septa) that are sparsely interspersed in the fiber cells could, however, have a function in the electrical conduction of excitation, corresponding functions are known from similar structures in other animal species. Rod-shaped inclusions could be endosymbiotic bacteria of unknown function. Contrary to previous assumptions, the appendages do not have any myofibrils , a role, analogous to muscle cells, in the movement of the animal is therefore rather unlikely. According to immunofluorescence observations, their surface bears the cadherin TaCDH. There is evidence that different Placozoa genetic lines contain different fiber cells.
As far as is known so far, there is no extracellular matrix as found in cnidarians or comb jellyfish as mesogloea or in sponges as mesohyl .
Unlike in tissue animals, pluripotent stem cells , i.e. those that can transform into other cell types, have not yet been clearly identified.
genetics
All the cell nuclei of the Placozoa cells contain twelve chromosomes, which are only about two to three micrometers in size . Three pairs are metacentric , the other acrocentric , that is, the centromere , the attachment point of the spindle fibers during cell division, is in the middle or on the outer edge of the respective chromosome. The cells of the fiber syncytium may be tetraploid , i.e. contain a four-fold set of chromosomes.
Overall, a single set of chromosomes from Trichoplax adhaerens contains less than 50 million base pairs (98 million base pairs of the diploid organism) and thus forms the smallest animal genome; the number of base pairs in the intestinal bacterium Escherichia coli is only a factor of 10 smaller. Between 11,000 and 12,000 protein- coding genes are estimated. In contrast to other small genome species, that of Trichoplax is not intron depleted; this is an indication against a subsequent reduction in size.
The genetic makeup of Trichoplax adhaerens has not yet been very well researched; however, some genes such as Brachyury or Tbx2 / 3 , which are homologous to corresponding base pair sequences in tissue animals, have already been detected. Of particular importance is Trox-2 , a Placozoa gene, which is presumably homologous to the genes known in cnidarians under the name Cnox-2 and in the bilaterally symmetrical Bilateria as Gsx ; it therefore belongs to the ParaHox genes. As a homeobox gene, which belongs to the Hox genes , it plays a role in the development of the embryo in tissue animals in the formation and body differentiation along the axis of symmetry; In the case of the cnidarians, for example, it determines the position of the mouth (oral) and the mouth (aboral) side of the animal. However, as mentioned, Placozoa have no axes of symmetry. In the Trichoplax body, the gene is read in a ring-shaped zone surrounding the body . A comparison of the gene arrangement of other genes on the chromosomal strand also provides indications that trochoplax may have had another, real Hox gene that was later lost - an indication of secondary simplification.
The Trichoplax genome also contains numerous other genes that are orthologous to genes for transcription factors that are involved in the formation of body axes in bilaterians (as well as humans), although these are not differentiated in Placozoa. There are genes for the Wnt signaling pathway and the BMP / TGF beta signaling pathway, which in Bilateria is involved in the development of the longitudinal axis and the dorsal-ventral axis. Although specialized nerve cells are not developed, proteins for neurotransmitters and ion channels typical for nerve cells are available. As is typical for multicellular cells (but also already present in many single-celled or colony-forming puffed-up flagellates ), genes for numerous cell adhesion molecules are found, including those that bind to molecules of the extracellular matrix , although this has not yet been proven with certainty in Trichoplax but available, but escaped previous detection methods.
distribution and habitat
Exact distribution information does not exist for the Placozoa, but the animals have been detected in the Red Sea , the Mediterranean , the Caribbean , off Hawaii , Guam , Samoa , Japan , Vietnam or Papua New Guinea and on the Great Barrier Reef off the east coast of Australia . As far as is known, they are widespread in all tropical and subtropical seas, north to the Mediterranean, at water temperatures between 10 and 32 ° C. Trichoplax adhaerens is also regularly "found" in seawater aquariums, for example in Plymouth in southern England or in Miami in the US state of Florida .
A direct search in the sea is impossible for the very small and inconspicuous animals. There are two methods of detection: either hard substrates such as stones are collected in the habitat and later examined more closely in the aquarium, or artificial substrates, usually glass slides , are exposed in the habitat, hoping for spontaneous colonization by the animals. The field finds so far come from shallow water of the sea coasts, down to a depth of about 20 meters, and tidal zones of tropical and subtropical seas, where the animals can be found on substrates such as trunks or roots of mangroves , mollusc shells, fragments of hard corals or simply on boulders . They can survive both brackish water- influenced habitats with a salinity of up to 2% as well as somewhat salted, concentrated seawater with a salinity of over 5%. Their frequency is higher in the summer months. According to model calculations, the frequency of occurrence increases with rising temperature and rising salinity, with nutrient-poor water being preferred. The different genetic lines (which presumably correspond to different species) show different preferences.
nutrition
Trichoplax adhaerens feeds on small algae , especially green algae (Chlorophyta) of the genus Chlorella , cryptomonads (Cryptophyta) of the genera Cryptomonas or Rhodomonas , cyanobacteria (Cyanobacteria) such as Phormidium inundatum , but also dead parts of other organisms. For this purpose, one or more small pockets are formed on the stomach side around the food particles, into which digestive enzymes are released by the gland cells; the animals temporarily develop an "outer stomach". The digested nutrients are absorbed by the flagella cells on the abdomen through pinocytosis ("cell drinking").
Food particles, including whole single-cell organisms, can also be ingested via the upper epitheloid (the animal's "back"). This diet is probably unique in the animal kingdom: food particles collected in a layer of mucus are pulled by fiber cells through intercellular gaps in the epithelium and digested by phagocytosis ("cell food"). This “collection” of food particles through an intact covering tissue is only possible because Placozoa lacks various “sealing” elements (a basal lamina under the epithelium and certain cell-cell connections).
Symbiotes
With this seemingly simplest animal, two different, very picky bacteria live in an intracellular symbiosis :
- The endoplasmic reticulum , a cell organelle of the fiber syncytium, is used to build proteins and membranes. This colonizes a bacterium belonging to the order of the Rickettsiales , provisionally named Candidatus Grellia incantans . Based on its gene expression , it is believed that it gets most of its nutrients from the host organism.
- Candidatus Ruthmannia eludens , a representative of the provisional Candidatus phylums Margulisbacteria, which has so far only been characterized according to sequence data and is probably closely related to the cyanobacteria , lives in cells with which the Trichoplax digests food. Ruthmannia eludens probably utilizes fats and other lipids from algae and can provide vitamins and amino acids to its host.
Locomotion
Placozoa can move in two different ways on a solid surface: On the one hand, their flagellated crawling sole enables them to glide slowly over the ground, on the other hand, like an amoeba , they can change their position by changing their body shape. The movements are not centrally coordinated because there is no muscle or nerve tissue.
A close connection between body shape and speed of movement could be demonstrated, which is also dependent on the food supply
- When the nutrient density is low, the cross-sectional area fluctuates slightly but irregularly, and the speed of movement is relatively constant at around 15 micrometers per second.
- On the other hand, if the nutrient density is high, the cross section oscillates with a stable period of around 8 minutes, whereby the largest dimension of the animal can be up to twice as large as the smallest. The speed of movement, which is consistently below 5 micrometers per second, fluctuates with the same period. A high speed always corresponds to a small cross-sectional area and vice versa.
Since the transition is not fluid but rather abrupt, the two modes of propagation can be very easily differentiated from one another. To simplify matters, Trichoplax adhaerens can be modeled as a non-linear dynamic system far from thermodynamic equilibrium .
A qualitative explanation for the behavior of the animal is as follows:
- When the nutrient density is low, Trichoplax maintains a constant speed to find food sources without wasting time.
- If such a source is identified by a high nutrient density, the individual concerned increases its cross-section at regular intervals and thus increases the contact area with the substrate. In this way, the area over which nutrients can be absorbed is expanded. At the same time, the animal reduces its speed in order to actually exhaust the available food supply.
- As soon as this is approximately done, Trichoplax reduces its cross-section again in order to move further. Because food sources such as algae carpets are often larger, it makes sense for an affected animal to stop moving after a short time in order to flatten itself out again and take in nutrients. Therefore, Trichoplax moves relatively slowly in this phase.
The specific direction in which Trichoplax moves is determined by chance: If you measure how fast an individual animal moves away from a (arbitrarily determined) starting point, you will find a linear relationship between the time passed and the mean square distance from the starting point. and current whereabouts. Such a connection also characterizes the random Brownian movement , which can thus serve as a model for the locomotion of the Placozoa.
Small animals are also able to actively swim with the help of their flagella. As soon as they touch a possible substrate, a dorsoventral reaction occurs : the flagella on the back continue to beat, while the flagella of the abdominal cells stop their beat rhythm. At the same time, the ventral side tries to make contact with the ground; Small cell protuberances and invaginations, the microvilli , which are located on the surface of the columnar cells on the ventral side, contribute to the attachment to the substrate through their adhesive (sticking) effect.
regeneration
A remarkable property of the Placozoa is that they can regenerate from the smallest of cells. Even if large parts of the organism are removed in the experiment, a complete animal will develop from the rest. It is also possible to pass Trichoplax adhaerens through a sieve so that the individual cells are not destroyed, but largely separated from one another. In the test tube they then come together again to form complete organisms. If this procedure is carried out with several previously colored animals at the same time, the same thing happens. In this case it can even happen that cells that previously belonged to a certain animal suddenly reappear as part of another.
Reproduction
Usually the Placozoa reproduce asexually. To do this, the animal constricts itself in the middle, so that two daughters of about the same size arise, which, however, remain loosely connected for a while after fission . Budding processes are less common: Small spheres of cells ("swarmers") floating in the water separate from the back, which combine all known cell types and subsequently grow into their own individual.
Sexual reproduction is possibly triggered by too high a population density, in some cases by high water temperatures; it has so far been a mystery and little understood in detail. In the laboratory, only degenerating individuals that swell up due to water intake form egg cells within four to six weeks. These are probably formed from cells of the lower epithelium and grow into the interior of the organism. As a result of the absorption of nutrients and (externally formed) yolk through phagocytosis from the dissolving maternal organism, they swell up to a size of 70–120 µm. Most individuals form only a single egg cell, rarely up to three. So-called F cells that are formed at the same time may represent the male sex cells ( sperm ), but their function has not been confirmed, and the fertilization process has not yet been observed; however, some proteins typical of sperm are expressed in the cells. After (assumed) fertilization, the egg cell forms the so-called fertilization membrane, a protective covering. Embryonic development begins with a complete, equal cleavage. The young embryos grow in the maternal organism, they are released through its degeneration and dissolution.
Despite considerable efforts, it has never been possible to keep embryos alive beyond the 128-cell stage. The further development is therefore unknown. Presumably a critical factor of the natural habitat that has not yet been understood is missing in the laboratory environment.
Because of the ability to clone indefinitely through asexual reproduction, the Placozoa's lifespan is potentially infinite; In practice, some lines of development that go back to a single animal have been kept in culture for a long time - in one case since 1969 - without the occurrence of sexual processes.
Tribal history, phylogeny
The ancestral relationships of the Placozoa are controversial. Fossil evidence does not exist and is not to be expected, so that the position of Trichoplax adhaerens has to be determined solely on the basis of the comparison of recent species. The Placozoa are next to the sponges (Porifera), the cnidarians (Cnidaria), the comb jellyfish (Ctenophora) and the bilateral animals (Bilateria) one of the five basic lines of development of the multicellular animals (known as the taxon Metazoa). The relationship between these five groups is scientifically controversial. Theoretically, there are 105 possibilities of parentage relationships for the ratio of 5 taxa, many of which have already been proposed as hypotheses. The results obtained so far, some of which initially appeared to be well established, turned out to be highly dependent on the method used and the number and composition of the taxa included in each case and are therefore all considered to be uncertain.
The assignment to the non- tissue animals ("Parazoa") is not based on the assumed relationships of the Placozoa, but instead classifies the animals according to their degree of organization: Just like the sponges (Porifera) with which they are united in this taxon, they lack tissue or Organs; the epitheloid is not regarded as a fully fledged tissue in this context. However, these are "primitive features", so-called symplesiomorphies , which go back to the common ancestral species of all animals and therefore, according to the phylogenetic system, can not establish any evolutionary relationship. Other biologists see regression in this, meaning that the animals would be secondary, simplified descendants of more complex, organized ancestors.
Placozoa as a sister group of the other Metazoa
Because of their simple structure, the Placozoa are sometimes viewed as model organisms for the development of single cells to multicellular cells . They are then considered the sister group of all other multicellular cells:
| Multicellular animals (Metazoa) |
|
||||||||||||
|
|
An important argument for such a basal position of the Placozoa is, in addition to their morphologically simple organization, the structure of the mitochondrial DNA , the independent genetic material of the mitochondria . As with many unicellular organisms, this is extremely complex, far more complicated than that of all other multicellular organisms, including the sponges. Phylogenomic studies in which the relationships were analyzed based on the comparison of homologous DNA sequences of the mtDNA, however, did not turn out to be very illuminating. Presumably the DNA of the mitochondria is no longer phylogenetically informative enough due to too many mutations. Genetic studies that showed a sister group relationship between the Placozoa and the other Metazoa are therefore considered to be very uncertain and hardly reliable.
Epitheliozoa hypothesis
The most important concept based on purely morphological properties sees the Placozoa as the closest relative of the tissue animals (Eumetazoa). The common taxon , known as Epitheliozoa, is in turn regarded as the sister group of the sponges (Porifera):
| Multicellular animals (Metazoa) |
|
||||||||||||||||||
|
|
Such a relationship is primarily supported by special cell-cell connections, the belt desmosomes , which occur not only in placozoa but in all animals outside the sponges and ensure that cells join together to form a seamless layer such as the epitheloid of placozoa can. The dorsal epitheloid is hypothesized to be homologous to the ectoderm , the ventral to the endoderm of the other metazoa. Trichoplax adhaerens also shares the gland cells that appear on the abdomen with most tissue animals. Both properties can be regarded as apomorphies , i.e. as evolutionarily derived characteristics, and thus establish a common taxon for all affected animals.
Eumetazoa hypothesis
A third hypothesis, primarily based on molecular genetics, sees the Placozoa as a greatly simplified tissue animal. According to this, Trichoplax adhaerens comes from animals with a much more complex structure that already had muscles and nerve tissue. Both types of tissue, as well as the basal lamina of the epithelium, have therefore only been lost through radical secondary simplification.
Various studies are currently coming to different results with regard to the exact sister group: Sometimes the Placozoa are considered the closest relatives of the cnidarians (Cnidaria), sometimes as the sister group of the rib jellyfish and occasionally they are even placed directly next to the bilaterally symmetrical Bilateria :
| Multicellular animals (Metazoa) |
|
||||||||||||||||||||||||
|
|
Merely a proposed classification in the cnidarians can currently be ruled out with a high degree of probability.
A critical objection to the proposed scenario is that it completely ignores the morphological characteristics of the animals. Such an extreme simplification, as it would have to be postulated for the Placozoa according to the model, is also only known from parasitic organisms, but it is difficult to explain functionally for a wild animal species such as Trichoplax adhaerens .
Systematics
Currently, only one species, Trichoplax adhaerens , is recognized as part of the Placozoa. However, in 1893 the Italian Francesco Saverio Monticelli described another species with the name Treptoplax reptans , which he found in the waters around Naples . However, it has been considered lost since 1896; today most zoologists question their existence.
Because there are often great genetic differences between representatives of Trichoplax adhaerens , which would lead to a division into different genera in other groups of organisms, it seems very likely that the only species according to morphological criteria actually corresponds to a group of cryptic , i.e. externally indistinguishable species. In fact, after electron microscopic examinations, the first morphological differences between different clones cultivated in the laboratory were also indicated. So far, around 200 different genetic lines, some with a wide geographical distribution, have been identified, which could possibly correspond to around 20-30 species. The problem of species delimitation in the group with few characteristics is currently still unsolved. The assignment of higher ranks of the rank-based (Linnaeus) taxonomy, such as families or orders, is currently generally not considered expedient.
Initial descriptions
- Placozoa
- Karl Gottlieb Grell: Trichoplax adhaerens FE Schulze and the origin of the metazoa. In: Naturwissenschaftliche Rundschau. 22, 1971. pp. 160-161. ISSN 0028-1050
- Treptoplax reptans
- Francesco Saverio Monticelli: Treptoplax reptans ng, n.sp. In: Atti della Reale Accademia dei Lincei, Quinta series, Rendiconti, Classe di scienze fisiche, matematiche e naturali 5, 1893, pp. 39-40. ISSN 0001-4435
- Trichoplax adhaerens
- Franz Eilhard Schulze: Trichoplax adhaerens, nov. gen., nov. spec. Zoologischer Anzeiger 6 (132), 1883: 92-97. Leipzig, Wilhelm Engelmann Verlag.
Web links
- Monitoring report with photos (English)
- Short description with photos (English)
Individual evidence
- ↑ a b Eitel, M. (2011): Trichoplax Schultze , 1883. In: Schierwater, B .; Eitel, M .; DeSalle, R. (2017). World Placozoa Database. Accessed through: World Register of Marine Species , accessed April 4, 2017.
- ^ Rüdiger Wehner, Walter Gehring: Zoologie. 24th edition, Thieme, Stuttgart, June 2007, p. 696
- ^ A b c Carolyn L. Smith, Frédérique Varoqueaux, Maike Kittelmann, Rita N. Azzam, Benjamin Cooper, Christine A. Winters, Michael Eitel, Dirk Fasshauer, Thomas S. Reese (2014): Novel Cell Types, Neurosecretory Cells, and Body Plan of the Early-Diverging Metazoan Trichoplax adhaerens. Current Biology 24: 1565-1572. doi : 10.1016 / j.cub.2014.05.046
- ↑ Loretta Guidi, Michael Eitel, Erica Cesarin, Bernd Schierwater, Maria Balsamo (2011): Ultrastructural Analyzes Support Different Morphological Lineages in the Phylum Placozoa Grell , 1971. Journal of Morphology 272: 371-378. doi : 10.1002 / jmor.10922
- ^ VJ Birstein (1989): On the karyotype of Trichoplax sp. (Placozoa). Biologisches Zentralblatt 108 (1): 63-67.
- ↑ a b Mansi Srivastava, Emina Begovic, Jarrod Chapman, Nicholas H. Putnam, Uffe Hellsten, Takeshi Kawashima, Alan Kuo, Therese Mitros, Asaf Salamov, Meredith L. Carpenter, Ana Y. Signorovitch, Maria A. Moreno, Kai Kamm, Jane Grimwood, Jeremy Schmutz, Harris Shapiro, Igor V. Grigoriev, Leo W. Buss, Bernd Schierwater, Stephen L. Dellaporta, Daniel S. Rokhsar (2008): The Trichoplax Genome and the Nature of Placozoans. Nature 454: 955-960. doi : 10.1038 / nature07191
- ↑ a b Olivia Mendivil Ramos, Daniel Barker, David EK Ferrier (2012): Ghost Loci Imply Hox and ParaHox Existence in the Last Common Ancestor of Animals. Current Biology 22 (20): 1951-1956. doi : 10.1016 / j.cub.2012.08.023
- ↑ a b Michael Eitel, Hans-Jürgen Osigus, Rob DeSalle, Bernd Schierwater (2013): Global Diversity of the Placozoa. PLoS ONE 8 (4): e57131 doi : 10.1371 / journal.pone.0057131
- ↑ Omid Paknia & Bernd Schierwater (2015): Global Habitat Suitability and Ecological Niche Separation in the Phylum Placozoa. PLoS ONE 10 (11): e0140162. doi : 10.1371 / journal.pone.0140162
- ↑ Harald R. Gruber-Vodicka, Nikolaus Leisch, Manuel Kleiner, Tjorven Hinzke, Manuel Liebeke, Margaret McFall-Ngai, Michael G. Hadfield, Nicole Dubilier: Two intracellular and cell type-specific bacterial symbionts in the placozoan Trichoplax H2. (2019). Nature Microbiology 4: 1465-1474. doi : 10.1038 / s41564-019-0475-9
- ↑ T. Ueda, S. Koga, YK Marayama: Dynamic patterns in the locomotion and feeding behavior by the placozoan Trichoplax adhaerens . In: BioSystems 54, 1999, 65-70. doi : 10.1016 / S0303-2647 (99) 00066-0
- ^ Karl G. Grell & Gertrud Benwitz (1981): Supplementary investigations on the ultrastructure of Trichoplax adhaerens FE Schulze (Placozoa). Zoomorphology 98 (1): 47-67.
- ↑ Michael Eitel, Loretta Guidi, Heike Hadrys, Maria Balsamo, Bernd Schierwater (2011): New Insights into Placozoan Sexual Reproduction and Development. PLoS ONE 6 (5): e19639. doi : 10.1371 / journal.pone.0019639
- ↑ Bernd Schierwater (2005): My favorite animal, Trichoplax adhaerens. BioEssays 27: 1294-1302.
- ↑ Bernd Schierwater, Peter WH Holland, David J. Miller, Peter F. Stadler, Brian M. Wiegmann, Gert Wörheide, Gregory A. Wray, Rob DeSalle (2016): Never Ending Analysis of a Century Old Evolutionary Debate: “Unringing” the Urmetazoon Bell. Frontiers in Ecology and Evolution 4: 5. doi : 10.3389 / fevo.2016.00005
- ↑ Martin Dohrmann & Gert Wörheide (2013): Novel Scenarios of Early Animal Evolution — Is It Time to Rewrite Textbooks? Integrative and Comparative Biology 53 (3): 503-511. doi : 10.1093 / icb / ict008
- ↑ Stephen L. DellaPorta, Anthony Xu, Sven Sagasser, Wolfgang Jakob, Maria A. Moreno, Leo W. Buss, Bernd Schierwater (2006): Mitochondrial genome of Trichoplax adhaerens supports Placozoa as the basal lower metazoan phylum. PNAS Proceedings of the National Academy of Sciences USA 103 (23): 8751-8756. doi : 10.1073 / pnas.0602076103
- ↑ Fernanda Britto da Silva, Valéria C. Muschner, Sandro L. Bonatto (2007): Phylogenetic position of Placozoa based on large subunit (LSU) and small subunit (SSU) rRNA genes. Genetics and Molecular Biology 30 (1): 127-132. doi : 10.1590 / S1415-47572007000100022
- ↑ Tetyana Nosenko, Fabian Schreiber, Maja Adamska, Marcin Adamski, Michael Eitel, Jörg Hammel, Manuel Maldonado, Werner EG Müller, Michael Nickel, Bernd Schierwater, Jean Vacelet, Matthias Wiens, Gert Wörheide (2013): Deep metazoan phylogeny: When different genes tell different stories. Molecular Phylogenetics and Evolution 67 (1): 223-233. doi : 10.1016 / j.ympev.2013.01.010
- ^ Peter Ax: Multicellular Animals: A new Approach to the Phylogenetic Order in Nature. Volume 1. Springer, Berlin / Heidelberg, 2012. ISBN 978 3642801143 . Epitheliozoa on page 77.
- ^ Franz Eilhard Schulze: Trichoplax adhaerens, nov. gen., nov. spec. Zoologischer Anzeiger 6 (132), 1883: 92-97 digitized online, at archive.org
