1,12-dodecanedioic acid
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 1,12-dodecanedioic acid | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 12 H 22 O 4 | |||||||||||||||
| Brief description |
white powder or flakes |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 230.31 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density | ||||||||||||||||
| Melting point |
126-129 ° C |
|||||||||||||||
| boiling point |
205 ° C (1 hPa) |
|||||||||||||||
| Vapor pressure |
<0.01 mbar (20 ° C) |
|||||||||||||||
| pK s value |
|
|||||||||||||||
| solubility |
0.04 g / l in water at 20 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
1,12-dodecanedioic acid , English 1,12-dodecanedioic acid ( DDDA ), is a long -chain linear dicarboxylic acid with terminal carboxy groups , which is particularly suitable as a monomer component for polyesters and polyamides with low water absorption due to its hydrophobic properties .
Manufacturing
1,12-dodecanedioic acid is produced on an industrial scale via a three-stage reaction sequence, starting from 1,5,9-cyclododecatriene (CDT), which is easily accessible from 1,3-butadiene .
First, CDT is hydrogenated practically quantitatively in the presence of Raney nickel at 200 ° C and 10–15 bar pressure to cyclododecane (CDAN), which is then oxidized with air or oxygen in the presence of boric acid at 150–160 ° C and normal pressure ( Bashkirov oxidation ).
The resulting cyclododecanol (CDOL) reacts to form cyclododecane triborate and is thus withdrawn from further oxidation to cyclododecanone (CDON). After hydrolysis of the triborate, the so-called ol-on mixture is obtained with a composition of 80 to 90% CDOL and 10-20% CDON.
Because of the low selectivity of the oxidation reaction, the reaction conversion is kept below 30%.
The resulting ol-on mixture is then either directly or after dehydration to the CDON with nitric acid and acetic acid as solubilizers in yields of about 75% of theory. oxidized to 1,12-dodecanedioic acid.
The oxidation of the ol-on mixture, catalyzed with copper powder and ammonium metavanadate , with 60% nitric acid at temperatures of 85 to 95 ° C. gives DDDA in a yield of 84% of theory.
The low selectivities of the oxidation reactions described have led to an intensive search for alternative routes that either bypass the air oxidation of cyclododecane to the ol-on mixture, e.g. B. proceed via the epoxidation of CDT or cyclododecene, which is hydroxylated to CDOL and can be further oxidized to DDDA.
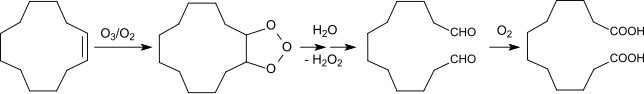
Cyclododecene can also be obtained directly by ozonolysis with useful yields (83% of theory) and high purity (99%) in z. B. propionic acid can be converted into 1,12-dodecadioic acid as an inerting solvent.
The dialdehyde dodecanedial, which is mainly obtained during ozonolysis with an ozone- oxygen mixture and subsequent hydrolysis of the ozonization products, is oxidized to DDDA by an oxidative-thermolytic aftertreatment with the ozone-free oxygen stream.
Another synthetic route is based on the reaction of cyclohexanone with hydrogen peroxide to form the peroxyhemiketal of cyclohexanone and subsequent reductive coupling with chelated iron (II) salts in an aqueous-organic phase mixture. This results in DDDA in up to 70% yield in addition to considerable amounts of caproic acid .
A completely different synthesis route starts with 10-undecenoic acid , which is easily accessible by pyrolysis of ricinoleic acid , which is hydroformylated in the presence of the catalyst carbonylhydridotris (triphenylphosphine) rhodium (I) and triphenylphosphine to 11-formylundecanoic acid in the presence of the catalyst customary for industrial hydroformylations and then hydroformylated in the presence of copper (II ) acetate and perpropionic acid with oxygen in a total yield of 70% of theory is oxidized to 1,12-dodecanedioic acid.
Using biotechnological methods, n-dodecane can be converted to DDDA with mutants of the Candida tropicalis yeast . The oxidation proceeds via the stages n-dodecanol, n-dodecanoic acid, 12-hydroxydodecanoic acid to DDDA.
The lauric acid (dodecanoic acid), which is abundantly available from the renewable raw materials coconut oil , bay oil and palm oil , can also be oxidized to DDDA by ω-oxidation with the help of genetically modified yeast strains.
In the laboratory, 1,12-dodecanedioic acid can also be prepared by oxidizing 1,12-dodecanediol with strong oxidizing agents such as B. an alkaline solution of potassium permanganate after recrystallization from ethanol / water 1:10 with a pure yield of 40 to 60% of theory. can be obtained.
Manufacturers of conventionally produced 1,12-dodecanedioic acid are the companies Evonik (DE), Hilead (CN), Invista (USA) and Ube (JP), of the biotechnologically produced DDDA Cathay Industrial Biotech (CN).
properties
1,12-dodecanedioic acid is a white, crystalline and odorless solid that dissolves very little in water at room temperature. In contrast, DDDA is soluble in short-chain alcohols such as methanol, ethanol, isopropanol, as well as in warm cyclohexanol and cyclohexanone, as well as acetone .
The high purity of the dicarboxylic acid building block DDDA required for polycondensation to achieve high molar masses of polyamides and polyesters can be achieved by heating in nitric acid, with aqueous sodium sulfite solution or in a mixture of acetic acid, phosphoric acid and hydrogen peroxide.
Applications
Polymers
The most important application is as a monomer component in polyamides and polyesters through polycondensation with diamines or diols . The most important polymer is polyamide 612 through polycondensation with 1,6-diaminohexane , which is used for highly stressed parts in vehicle construction due to its high melting point (218 ° C), low water absorption and good hydrolysis, chemical and weather resistance. Manufacturers of PA 612 are Dupont (Zytel®), EMS-Chemie (Grilamid 2d®), Evonik (Vestamid D®) and Ube (Ubesta®).
In the 1970s, Dupont marketed silk-like polyamide textile fibers made from DDDA and trans, trans-bis (4-aminocyclohexyl) methane under the brand name Qiana®, which were particularly soft and high-gloss due to their trilobal fiber cross-section, but were lower than conventional nylon fibers Exhibited tear strengths and could not establish themselves on the market.
In contrast to polyamides made from DDDA, polyesters have relatively low melting points (70 to 90 ° C) and their properties are similar to polyolefin polyethylene .
Ester
Diesters of DDDA with lower alcohols such as methanol and isopropanol , like macrocyclic ketones or lactones of similar chain length, have fragrance properties that, in the case of dimethyl ester, are “nice like musk, hot aldehyde, like a blown candle, slightly woody” and with diisopropyl ester as “like musk, warm , after a blown candle, weakly woody ”are described.
Diesters with higher alcohols, such as. B. 2-ethylhexanol or isotridecanol are odorless and can be used as a solvent for perfume compositions.
Diesters of 1,12-dodecanedioic acid are also used as synthetic lubricants and plasticizers.
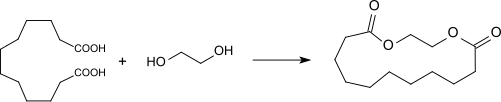
The cyclic ester made from DDDA and ethylene glycol also smells musky and is a very good fixative in perfume production.
O derivatives
Catalytic hydrogenation of DDDA or its diesters leads to 1,12-dodecanediol, which is suitable as a long-chain diol for the production of polyesters, polyurethanes, polycarbonates and epoxy resins.
N derivatives
By reaction with ammonia and subsequent elimination of water with dodecanedinitrile as solvent in the presence of 85% orthophosphoric acid at reaction temperatures of up to 345 ° C. in yields of up to 93% of theory, DDDA is obtained . Dodecanedinitrile obtained by catalytic hydrogenation in the u. a. 1,12-dodecanediamine, which is of interest as a diamine for polyamide or polyurethane syntheses, can be converted.
DDDA is also used in adhesives and hot-melt adhesives, corrosion inhibitors, surfactants, electrolytes for capacitors, as crosslinkers in powder coatings, etc.
Individual evidence
- ↑ a b c d e f g h Entry on dodecanedioic acid in the GESTIS substance database of the IFA , accessed on September 21, 2014(JavaScript required) .
- ↑ a b c d e f Invista, Technical Information C12, Dodecanedioic Acid (DDDA) ( Memento from October 6, 2014 in the Internet Archive ), accessed on October 1, 2014.
- ↑ a b Data sheet dodecanedioic acid (PDF) from Merck , accessed on October 1, 2014.
- ↑ G. Oenbrink, T. Schiffer: Ullmann's Encyclopedia of Industrial Chemistry, 6th Edition . Cyclododecanol, Cyclododecanone and Laurolactam. Wiley-VCH, 2000, doi : 10.1002 / 14356007.a08_201.pub2 .
- ^ Ullmann's Fine Chemicals: Dicarboxylic Acids, Aliphatic . Wiley-VCH, 2014, ISBN 978-3-527-33477-3 , pp. 592 .
- ↑ Invista, C12 High Performance Intermediates, C12Process ( Memento from October 6, 2014 in the Internet Archive )
- ↑ a b H.-J. Arpe: Industrial Organic Chemistry . 6., completely revised Edition. Wiley-VCH, 2007, ISBN 978-3-527-31540-6 .
- ↑ Patent US3087963 : Preparation of 1,12-dodecanedioic acid. Published April 30, 1963 , Applicant: Esso Research and Engineering Co., Inventor: HK Wiese, SB Lippincott.
- ↑ a b Patent US3903152 : Process for producing highly pure 1,12-dodecanedioic acid. Applied on June 29, 1973 , published on September 2, 1975 , Applicants: Toagosei Chemical Industry C. Ltd., Inventors: T. Matsubara, Y. Ishibashi, Y. Okada.
- ↑ Patent EP2407444 : Process for the preparation of dodecanedioic acid. Registered on March 18, 2009 , published on January 18, 2012 , applicant: Invista Technologies Sarl, inventor: G. Rajendran.
- ↑ TM Oshnyakova, NA Shchadneva, RI Khusnutdinov, UM Dzhemilev: Addition of Water and Carbon Tetrachloride to Cyclododecene in the Presence of Chromium Catalysts . In: Russ. J. Org. Chem. Volume 44 , no. 8 , 2008, ISSN 1070-4280 , p. 1240-1242 .
- ↑ Patent DE2942279A1 : Process for converting olefins in a carboxylic acid medium with ozone. Registered on October 19, 1979 , published on April 30, 1981 , applicant: Chemische Werke Hüls AG, inventor: KD Dohm, P. Hofmann.
- ↑ Patent WO03022792A1 : One step process for producing dicarboxylic acids. Filed on September 4, 2002 , published on March 20, 2003 , Applicant: EI Du Pont de Nemours and Co., Inventor: CA Thayer II.
- ↑ a b Patent US3907883 : Process for production of 1,12-dodecanedioic acid. Published on September 23, 1975 , Applicant: EI Du Pont de Nemours and Co., Inventor: DE Welton.
- ↑ Patent EP0258535 : Process for the production of 1,12-dodecanedioic acid II. Registered on May 19, 1987 , applicant: Degussa AG, inventor: J. Andrade, K. Köhler, G. Prescher.
- ↑ Z.-H. Yi, H.-J. Rehm: Metabolic formation of dodecanedioic acid from n-dodecane by a mutant of Candida tropicalis . In: Europ. J. Appl. Microbiol. Biotechnol. tape 14 , no. 4 , 1982, pp. 254-258 , doi : 10.1007 / BF00498473 .
- ↑ a b K. Kroha: Industrial biotechnology provides opportunities for commercial production of new long-chain dibasic acids . In: inform . tape 15 , no. 9 , 2004, p. 568-571 ( PDF ).
- ↑ Patent WO2013006730 : Biological methods for preparing a fatty dicarboxylic acid. Filed July 5, 2012 , published January 10, 2013 , Applicant: Verdezyne, Inc., Inventors: T. Beardslee, S. Picataggio, ED Eirich, JM Laplaza.
- ↑ NN: 6.1.2.1. Oxidation of 1,12-dodecanediol with potassium permanganate to dodecanedioic acid (1) . March 9, 2007 ( ioc-praktikum.de [PDF]).
- ↑ Dibasic Acids. In: Cathay Industrial Biotech. Cathay Industrial Biotech, accessed March 15, 2019 .
- ↑ Patent US3714244 : Method for purifying 1,12-dodecanedioic acid. Applied on April 6, 1971 , published January 30, 1973 , Applicants: Toagosei Chemical Industry Co. Ltd., Inventors: Y. Okada, T. Matsubara.
- ↑ Patent US4149013 : Process for purifying 1,12-dodecanedioic acid. Applied on May 19, 1978 , published April 10, 1979 , applicant: EI Du Pont de Nemours and Co., inventor: DA Klein.
- ↑ Evonik Industries: Vestamid D - polyamide 612
- ^ HG Elias: Macromolecules: Volume 3: Industrial polymers and syntheses, 6th edition . Wiley-VCH, 2001, ISBN 3-527-29962-9 , pp. 185 .
- ^ G. Barbiroli, C. Lorenzetti, C. Berti, M. Fiorini, P. Manaresi: Polyethylene like polymers. Aliphatic polyesters of dodecanedioic acid: 1. Synthesis and properties . In: Eur. Polym. J. Band 39 , no. 4 , 2003, p. 655-661 , doi : 10.1016 / S0014-3057 (02) 00280-X .
- ↑ Patent EP0103125 : Aliphatic dicarboxylic acid esters as fragrances and perfume compositions and perfumed products containing them. Registered on July 27, 1983 , published on October 21, 1987 , applicant: Haarmann & Reimer GmbH, inventors: W. Sturm, G. Mansfeld, H. Reindl.
- ↑ Patent EP1707185 : Solvent materials and methods for preparing fragrance compositions. Filed January 26, 2006 , published October 2, 2006 , Applicant: International Flavors & Fragrances, Inc., Inventors: APS Narula, AT Levorse, Jr., JS Huang.
- ↑ Patent US5717111 : Process for the continuous preparation of macrocyclic compounds. Registered on March 8, 1996 , published on February 10, 1998 , applicant: Hüls AG, inventor: G. Köhler, M. Feld, J. Metz.
- ↑ Invista: C12 High Performance Intermediates, Macrocyclics, Cyclic Esters ( Memento from October 6, 2014 in the Internet Archive )
- ^ Condea: Dr. Z presents All about fatty alcohols, Saturated Fatty Alcohols ( Memento of September 27, 2007 in the Internet Archive ), 2000.
- ↑ Patent US3707546 : Process for preparing dodecanedinitrile. Applied on April 2, 1971 , published December 26, 1972 , applicant: EI Du Pont de Nemours and Co., inventor: DA Klein.
- ↑ Patent US3987099 : Process for the hydrogenation of dodecanedioic acid dinitrile. Registered on October 24, 1974 , published on October 19, 1976 , applicant: Chemische Werke Hüls AG, inventor: G. Hockele, G. Ludwig.