Cannabinoids
Cannabinoids are transformation products and synthetic analogues of some terpene phenols that were mainly found in the hemp plant ( Cannabis sativa or Cannabis indica ). Research into cannabinoids led to the discovery of the endocannabinoid system . The body's own substances that have similar pharmacological properties are called endocannabinoids . Recent research shows that other plants also produce phytocannabinoids, which act on the endocannabinoid system just like the cannabinoids of the hemp plant.
Cannabinoids are used medicinally in various areas of application, such as neuropathic pain and spasticity ( preparations made from cannabis flowers ), loss of appetite in HIV / AIDS , nausea and vomiting during chemotherapy ( nabilone , dronabinol ), and certain forms of childhood epilepsy ( cannabidiol ).
Phytocannabinoids
Phytocannabinoids from the hemp plant
The hemp plant C. sativa contains at least 113 phytocannabinoids from the group of terpene phenols, which have not yet been discovered in any other plant. The most studied cannabinoid is Δ 9 -tetrahydrocannabinol (Δ 9 -THC), which was isolated in 1964 by Yehiel Gaoni and Raphael Mechoulam at the Weizmann Institute for Science in Israel. Cannabinoid acids as precursors of neutral cannabinoids were known in the 1950s for their antibiotic effects and were e.g. B. used in Czechoslovakia in veterinary medicine. Cannabidiol (CBD), another low-psychoactive cannabinoid, is being studied for its anti-inflammatory, anti-schizophrenic, and anti-epileptic properties. Most other cannabinoids have been tested for psychoactivity .
Some phytocannabinoids from the cannabis plant:
| Cannabinoid type | number | Cannabinoid type | number | Cannabinoid type | number | Cannabinoid type | number |
|---|---|---|---|---|---|---|---|
| Δ 9 -tetrahydrocannabinol | 9 | Δ 8 -tetrahydrocannabinol | 2 | Δ 9 -tetrahydrocannabivarin | - | Cannabidiol | 7th |
| Cannabigerol | 6th | Cannabichromes | 5 | Cannabicyclol | 3 | Cannabielsoin | 5 |
| Cannabitriol | 9 | Cannabinol | > 1 | Cannabinodiol | > 1 | Various | 11 |
Cannabis also contains a wide variety of non-cannabinoids, over 120 different terpenes, and 21 flavonoids with different pharmacological properties. There is evidence that cannabinoids such as cannabinol (CBN), cannabidiol (CBD) and others modify the effects of Δ 9 -THC.
The cannabinoids contained in cannabis have partially opposite effects: some cannabinoids are a. Agonists of the CB 1 / CB 2 receptors , while others either express no affinity or are antagonists .
The proportions of cannabinoids present in cannabis vary greatly depending on factors such as variety / genetics, storage conditions or geographical origin.
Phytocannabinoids of the hemp plant as a tabular overview
| Phytocannabinoids from the hemp plant | ||||
|---|---|---|---|---|
| Cannabigerol-like (CBG) | ||||

Cannabigerol |

Cannabigerol Monomethylether |
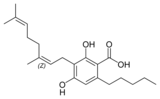
Cannabinerolic acid A |

Cannabigerovarin |
|
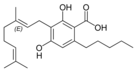
Cannabigerolic acid A |

Cannabigerolic acid A monomethyl ether |

Cannabigerovaric Acid A |
||
| Cannabichrome-like (CBC) | ||||

(±) - Cannabichromene |
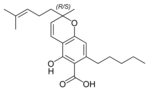
(±) - Cannabichromic acid A |
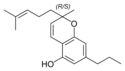
(±) - cannabichromes , (±) - cannabichromevarin |

(±) - Cannabichromevaric Acid A |
|
| Cannabidiol-like (CBD) | ||||
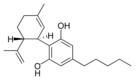
(-) - Cannabidiol |

Cannabidiol Monomethylether |
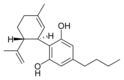
Cannabidiol-C 4 |
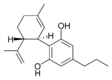
(-) - Cannabidivarin |

Cannabidiorcol |
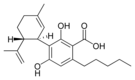
Cannabidiolic |

Cannabidivaric |
|||
| Cannabinodiol-like (CBND) | ||||

Cannabinodiol |
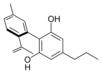
Cannabinodivarin |
|||
| Tetrahydrocannabinol-like (THC) | ||||
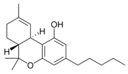
Δ 9 - tetrahydrocannabinol |

Δ 9 - Tetrahydrocannabinol-C 4 |

Δ 9 - Tetrahydrocannabivarin |

Δ 9 - Tetrahydrocannabiorcol |
|

Δ 9 - tetrahydro- |
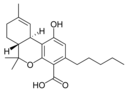
Δ 9 - tetrahydro- |

Δ 9 -Tetrahydro- |
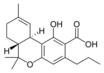
Δ 9 - tetrahydro- |

Δ 9 -Tetrahydro- |

(-) - Δ 8 - trans - (6a R , 10a R ) - |

(-) - Δ 8 - trans - (6a R , 10a R ) - |

(-) - (6a S , 10a R ) -Δ 9 - |
||
| Cannabinol-like (CBN) | ||||
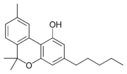
Cannabinol |

Cannabinol-C 4 |

Cannabivarin |
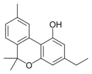
Cannabinol-C 2 |

Cannabiorcol |
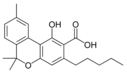
Cannabinolic acid A |
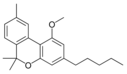
Cannabinol |
|||
| Cannabitriol-like (CBT) | ||||
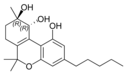
(-) - (9 R , 10 R ) - trans - |
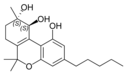
(+) - (9 S , 10 S ) - cannabitriol |

(±) - (9 R , 10 S / 9 S , 10 R ) - |

(-) - (9 R , 10 R ) - trans - |

(±) - (9 R , 10 R / 9 S , 10 S ) - |
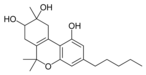
8,9-dihydroxy-Δ 6a (10a) - |

Cannabidiolic |

(-) - (6a R , 9 S , 10 S , 10a R ) - |

(-) - 6a, 7,10a-trihydroxy- |
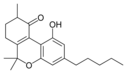
10-Oxo-Δ 6a (10a) - |
| Cannabielsoin-like (CBE) | ||||
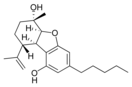
(5a S , 6 S , 9 R , 9a R ) - |
(5a S , 6 S , 9 R , 9a R ) - |
|||

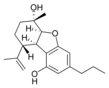
(5a S , 6 S , 9 R , 9a R ) - |

(5a S , 6 S , 9 R , 9a R ) - |

(5a S , 6 S , 9 R , 9a R ) - |
||

Cannabiglendol-C 3 |

Dehydrocannabifuran |

Cannabifuran |
||
| Isocannabinoids | ||||

(-) - Δ 7 - trans - (1 R , 3 R , 6 R ) - |
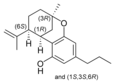
(±) -Δ 7 -1,2- cis - |
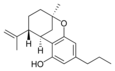
(-) - Δ 7 - trans - (1 R , 3 R , 6 R ) - |
||
| Cannabicyclol-like (CBL) | ||||

(±) - (1a S , 3a R , 8b R , 8c R ) - |

(±) - (1a S , 3a R , 8b R , 8c R ) - |
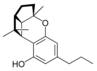
(±) - (1a S , 3a R , 8b R , 8c R ) - |
||
| Cannabicitran-like (CBT) | ||||
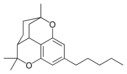
Cannabicitran |
||||
| Cannabichromanone-like (CBCN) | ||||

Cannabichromanone |

Cannabichromanone-C 3 |

Cannabicoumaronone |
||
Phytocannabinoids from other plants
Researchers at the Swiss Federal Institute of Technology in Zurich showed in 2006 that N -isobutylamides from Echinacea represent a new class of potent cannabinoid mimetics that bind to the peripheral CB 2 cannabinoid receptors on immune cells, but not to the CB 1 receptors in the central one Nervous system. Thus, Cannabis sativa is not the only plant that makes cannabinoid receptor ligands . Beta- caryophyllene occurs in various aromatic plants and is also a CB 2 cannabinoid. Yangonin from the kava plant (Piper methysticum) and various catechins from the tea plant (Camellia sinensis) also act as CB 1 receptor agonists.
Endocannabinoids
Anandamide , 2-arachidonylglycerol , O -arachidonylethanolamide , N -arachidonoyldopamine, γ-linolenoylethanolamide, docosatetraenoylethanolamide and 2-arachidonylglyceryl ether are endogenous cannabinoids (endocannabinoids) which act as neurosurgery endocannabinoids.
Synthetic cannabinoids
Artificial cannabinoids can be produced semi-synthetically, i.e. H. from natural cannabinoids, as well as fully synthetic from simple raw materials. Synthetic cannabinoids are used medicinally and are used in neuroscience to understand how cannabinoids work in the brain. They are also used in herbal mixtures as legal cannabis substitutes . Some synthetic cannabinoids are e.g. B.
| CP-55,940: Synthesized 1974, 40-50x as potent as Δ 9 -THC | CP-47,497 : ( proven to be the main active ingredient in the fashion drug " Spice ") | HU-210 : 100–800 times the potency based on THC, is said to have a cell growth-promoting and antidepressant effect according to animal experiments | HU-211 : is the enantiomer of HU-210 |
| HU-308 | HU-331 | RCS-4 | RCS-8 |
| SR-141716A : Is a selective CB 1 - antagonist and was admitted briefly for weight reduction as drugs. It is also being studied as a smoking cessation agent. | Nabilone : Used in oncology to treat the side effects of chemotherapy as an antiemetic . | 9-nor-9beta-hydroxyhexahydrocannabinol (Beta-HHC) | JWH-015: Research Chemical; triggers cell death in thymocytes . A possible immunosuppressant . |
| JWH-018 : Detected as an active ingredient in the fashion drug " Spice " | JWH-019 : Detected as an active ingredient in the fashion drug "Spice" | JWH-073 : Detected as an active ingredient in the fashion drug "Spice" | JWH-081 |
| JWH-122 : Detected in smoking mixtures | JWH-133: Research Chemical; shows anti-inflammatory and anti-cancer properties in animal models . | JWH-200 | JWH-203 |
| JWH-210 | JWH-250 | JWH-251 | JWH-398 |
| AM-2201 : Detected in smoking mixtures | AM-694 | CB-25 | CB-52 |
| WIN 55,212-2 | WIN 55,212-3 | 5F-MDMB-PICA |
Analysis of cannabinoids
The coupling of HPLC and mass spectrometry ( HPLC-MS ) after extraction of the sample material can be used for reliable analysis of the cannabinoids . For the reliable identification and quantification of AM 694 and its metabolites in biological material, the SPE with subsequent GC-MS or HPLC-MS can be used for sample preparation .
Legal position
- Germany
Some cannabinoids are the Narcotics Act assumes or covered by the law to combat the spread of new psychoactive substances and are therefore limited or only by prescription as a drug available.
- Switzerland
In Switzerland , the doctor must apply for a patient-specific exemption from the Federal Office of Public Health (FOPH) for therapy with dronabinol . Since dronabinol is not a compulsory health insurance service, the assumption of costs must be clarified in advance and on a case-by-case basis; with some health insurances you need additional insurance. Over 500 patients with amyotrophic lateral sclerosis , anxiety disorders , epilepsy , Crohn's disease , Parkinson's disease , polyarthritis , restless legs syndrome , Tourette's syndrome or tumor pain benefit from a medical prescription for cannabidiol. Multiple sclerosis sufferers can take the prescription drug Savitex, which contains CBD and THC, to relieve cramps. The Federal Office of Public Health assumes that around 100,000 people illegally use cannabis products for self-medication.
Since 2011, cannabis cultivation with a THC content of up to 1% has been permitted in Switzerland, mainly because of the natural fluctuations in hemp plants; previously the limit was 0.3%, but it could not be adhered to on a regular basis.
literature
- Roger Pertwee (Ed.): Cannabinoids. (= Handbook of Experimental Pharmacology. Volume 168). Springer, Berlin / Heidelberg 2005, ISBN 3-540-22565-X .
- Roger Pertwee (Ed.): Endocannabinoids. (= Handbook of Experimental Pharmacology. Volume 231). Springer, Berlin / Heidelberg 2015, ISBN 978-3-319-20825-1 .
- Franjo Grotenhermen (ed.): Cannabis and cannabinoids. Pharmacology, toxicology and therapeutic potential. Verlag Hans Huber, Bern / Göttingen / Toronto / Seattle 2004, ISBN 3-456-84105-1 .
- Vincenzo Di Marzo (Ed.): Cannabinoids. Wiley Blackwell, 2014, ISBN 978-1-118-45129-8 .
- B. Chakravarti, J. Ravi, RK Ganju: Cannabinoids as therapeutic agents in cancer: current status and future implications. In: Oncotarget. Volume 5, Number 15, August 2014, pp. 5852-5872. PMID 25115386 .
Web links
- Bela Szabo: Pharmacology of Cannabinoid Receptors . (PDF; 1.23 MB) In: Biotrend Reviews. No. February 02, 2008; BIOTREND Chemicals AG
- Marc Steffens: Modulation of the neocortical neurotransmission by exogenous and endogenous cannabinoids taking into account possible species differences between humans and animals . (PDF; 1.24 MB) uni-freiburg.de, dissertation
Individual evidence
- ^ A b S. Raduner, A. Majewska, JZ Chen, XQ Xie, J. Hamon, B. Faller, KH Altmann, J. Gertsch: Alkylamides from Echinacea are a new class of cannabinomimetics. Cannabinoid type 2 receptor-dependent and -independent immunomodulatory effects. In: The Journal of biological chemistry. Volume 281, Number 20, May 2006, pp. 14192-14206, doi: 10.1074 / jbc.M601074200 . PMID 16547349 .
- ^ A b A. Ligresti, R. Villano, M. Allarà, I. Ujváry, V. Di Marzo: Kavalactones and the endocannabinoid system: the plant-derived yangonin is a novel CB? receptor ligand. In: Pharmacological Research . Volume 66, Number 2, August 2012, pp. 163-169, doi: 10.1016 / j.phrs.2012.04.003 . PMID 22525682 .
- ^ A b G. Korte, A. Dreiseitel, P. Schreier, A. Oehme, S. Locher, S. Geiger, J. Heilmann, PG Sand: Tea catechins' affinity for human cannabinoid receptors. In: Phytomedicine . Volume 17, number 1, January 2010, pp. 19-22, doi: 10.1016 / j.phymed.2009.10.001 . PMID 19897346 .
- ↑ Oier Aizpurua-Olaizola, Umut Soydaner, Ekin Öztürk, Daniele Schibano, Yilmaz Simsir, Patricia Navarro, Nestor Etxebarria, Aresatz Usobiaga: Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes ACS Publications, J. . Prod., 2016, 79 (2), pp. 324-331, doi: 10.1021 / acs.jnatprod.5b00949 .
- ^ Y. Gaoni, R. Mechoulam: Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. In: Journal of the American Chemical Society. 86, 1964, p. 1646, doi: 10.1021 / ja01062a046 .
- ^ R. Mechoulam: Plant cannabinoids: a neglected pharmacological treasure trove. In: British Journal of Pharmacology . Volume 146, Number 7, December 2005, pp. 913-915, doi: 10.1038 / sj.bjp.0706415 . PMID 16205721 , PMC 1751232 (free full text).
- ^ Roger G. Pertwee: Pharmacological and therapeutic targets for Δ⁹-tetrahydrocannabinol and cannabidiol. In: Euphytica. 140, 2004, p. 73, doi: 10.1007 / s10681-004-4756-9 .
- ↑ a b probably oxidation artifacts of tetrahydrocannabinol or cannabidiol.
- ↑ Table of Natural Cannabinoids
- ↑ J. Gertsch, M. Leonti, S. Raduner, I. Racz, JZ Chen, XQ Xie, KH Altmann, M. Karsak, A. Zimmer: Beta-caryophyllene is a dietary cannabinoid. In: Proceedings of the National Academy of Sciences . Volume 105, Number 26, July 2008, pp. 9099-9104, doi: 10.1073 / pnas.0803601105 . PMID 18574142 , PMC 2449371 (free full text).
- ^ R. Mechoulam, LA Parker: The endocannabinoid system and the brain. In: Annual review of psychology. Volume 64, 2013, pp. 21-47, doi: 10.1146 / annurev-psych-113011-143739 . PMID 22804774 (Review).
- ↑ AC Porter, JM Sauer et al .: Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. In: The Journal of pharmacology and experimental therapeutics. Volume 301, Number 3, June 2002, pp. 1020-1024. PMID 12023533 .
- ↑ a b c d Bernd Dicks: First analysis: the fashion drug Spice contains hashish-like active ingredient. In: Spiegel Online . December 15, 2008, accessed June 4, 2015 .
- ↑ Marilyn A. Huestis: Synthetic Cannabinoids, Forensic & Legal Aspects . ( Memento of the original from March 22, 2016 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF) PhD, National Institutes of Health
- ↑ Fashion drug: Spice's main active ingredient discovered. In: Frankfurter Rundschau . January 19, 2009, accessed July 1, 2012 .
- ↑ C. Lombard, M. Nagarkatti, P. Nagarkatti: CB2 cannabinoid receptor agonist, JWH-015, triggers apoptosis in immune cells: potential role for CB2-selective ligands as immunosuppressive agents. In: Clinical immunology (Orlando, Fla.). Volume 122, number 3, March 2007, pp. 259-270, doi: 10.1016 / j.clim.2006.11.002 . PMID 17185040 . PMC 1864948 (free full text).
- ↑ a b Stefan Kneisel, Folker Westphal among others: Trends in the field of synthetic cannabinoid mimetics: mass spectra and ATR-IR spectra of new compounds from the period from the end of 2010 to the end of 2011 . (PDF) In: Toxichem Krimtech. 78 (3), 2011, p. 465.
- ↑ C. Blázquez, ML Casanova, A. Planas, T. Gómez Del Pulgar, C. Villanueva, MJ Fernández-Aceñero, J. Aragonés, JW Huffman, JL Jorcano, M. Guzmán: Inhibition of tumor angiogenesis by cannabinoids. In: The FASEB Journal : official publication of the Federation of American Societies for Experimental Biology. Volume 17, Number 3, March 2003, pp. 529-531, doi: 10.1096 / fj.02-0795fje . PMID 12514108 .
- ↑ H. Xu, CL Cheng et al: Anti-inflammatory property of the cannabinoid receptor-2-selective agonist JWH-133 in a rodent model of autoimmune uveoretinitis. In: Journal of Leukocyte Biology . 82, 2007, pp. 532-541, doi: 10.1189 / jlb.0307159 .
- ↑ Warning cannabis with synthetic cannabinoids. February 13, 2020, accessed March 6, 2020 .
- ↑ 5-fluoro MDMB-PICA (CAS 1971007-88-1). Retrieved March 6, 2020 .
- ^ S. Kneisel, V. Auwärter: Analysis of 30 synthetic cannabinoids in serum by liquid chromatography-electrospray ionization tandem mass spectrometry after liquid-liquid extraction. In: J Mass Spectrom . 47 (7), Jul 2012, pp. 825-835. PMID 22791249 .
- ^ E. Bertol, F. Vaiano, MG Di Milia, F. Mari: In vivo detection of the new psychoactive substance AM-694 and its metabolites. In: Forensic Sci Int. 256, Nov 2015, pp. 21-27. PMID 26295909 .
- ↑ Therapy with cannabinoids ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. , Hänseler AG, accessed October 5, 2011.
- ↑ Information on dronabinol and cannabis at www.panakeia.ch ( Memento of the original from July 20, 2017 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Simon Christen: Is the pharmaceutical industry afraid of the success of cannabis? DOK SRF, Zurich April 7, 2017
- ↑ Rebekka Haefeli: Now comes cannabis light. A huge disappointment for stoners, the great hope for planters: low-THC cannabis. The trade in the herb could develop into a billion dollar business . Observer, Zurich, July 7, 2017, pp. 36–40.