Calcium carbide
| Crystal structure | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
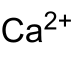 |
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Calcium carbide | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Ratio formula | CaC 2 | ||||||||||||||||||
| Brief description |
colorless crystals |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 64.10 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
2.22 g cm −3 |
||||||||||||||||||
| Melting point |
2160 ° C |
||||||||||||||||||
| boiling point |
2300 ° C |
||||||||||||||||||
| solubility |
Calcium carbide is not completely soluble in any solvent |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
0.14 mg m −3 |
||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
−59.8 kJ / mol |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Calcium carbide , and calcium acetylide , the calcium - salt of ethyne and a acetylide . In its pure state it is a white solid.
history
Calcium carbide was first presented by Edmund Davy in 1836 and by Friedrich Wöhler in 1862 and described in detail by Marcellin Berthelot in 1862 . The laboratory calcium carbide production in the electric furnace was carried out in 1892 by Thomas Willson in America and by Henri Moissan in Paris. The industrial calcium carbide extraction began in 1895 in the aluminum industry AG in Neuhausen in Switzerland; it was included in Norway and Germany ( Aluminum Industrie Aktiengesellschaft in Rheinfelden, Baden ) in 1898 . In the US, production began around the same time in the predecessor organizations of Union Carbide , to which Willson sold his patent in 1895, and in Canada through companies founded by Willson. Annual production fell from 10 million tons in the 1960s to 2 million tons in 2010.
Manufacturing
Technically, calcium carbide is obtained in smelting reduction furnaces (a special form of the electric arc furnace ) at 2000 to 2300 ° C from calcium oxide (quicklime) and coke . Due to the high power consumption of these ovens, production is very costly. Operation is therefore only profitable where both raw materials and electricity can be obtained cheaply, such as from hydropower plants in water-rich regions.
The resulting brown lumps contain 80 to 85 percent calcium carbide, the rest consists of impurities such as calcium oxide, calcium phosphide , calcium sulfide , calcium nitride or silicon carbide .
A process developed in 2010 aims to replace some of the coal and coke used with plastic waste (KBK) as a secondary raw material.
Purer calcium carbide can be obtained by reversing the reaction to form calcium cyanamide or by heating a mixture of calcium cyanamide and carbon in a high vacuum.
The representation from the elements is also possible at 1250 ° C.
properties
Physical Properties
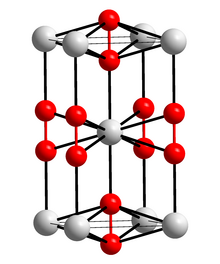
In its pure state, calcium carbide is a colorless, crystalline mass. There are two modifications , the tetragonal and a face-centered cubic modification of the pyrite type, which is formed by heating above 440 ° C.
The calcium carbide on the market is colored gray to gray-black due to the added carbon components or brown due to iron oxide impurities; In addition, it contains some calcium oxide , calcium phosphide , calcium sulfide , ferrosilicon , magnesium nitride and silicon carbide due to its production , so that on average it only has a CaC 2 content of 82%.
Chemical properties
Calcium carbide belongs in the group of carbides to the acetylides, as it is formally derived from ethyne . Calcium carbide is not soluble in any solvent (unchanged). If it comes into contact with water , it decomposes in a vigorous reaction (hydrolysis) to ethyne and calcium hydroxide .
The unpleasant “carbide” smell in this reaction is not caused by the ethyne gas that is formed, but is due to gases such as monophosphine , ammonia and hydrogen sulfide , which are produced when the impurities react with water. This is how monophosphine is formed through the hydrolysis of calcium phosphide Ca 3 P 2 contained in calcium carbide :
At temperatures above 905 ° C it reacts with nitrogen to form calcium cyanamide ; pressure hydrogenation supplies calcium hydride ; its reducing properties are also used technically.
use
In common parlance, carbide is usually equated with calcium carbide . In the gas generator, it reacts with water to form ethine (common name: acetylene), which is used for various applications.
- In the past, calcium carbide was mixed with water in carbide lamps , with which it reacted to form combustible ethine ; the ethine was kindled and burned with a bright flame. This method was used in particular in the mine lamps in underground mining. Carbide lamps are still preferred by speleologists today because they give a particularly beautiful light and the flame can be used to mark surfaces with soot .
- Manufacture of calcium cyanamide
- Synthesis of ethyne, operation of carbide developers (only important in emerging countries today)
- For desulphurising pig iron and liquid steel in the steel industry
- From around 1900 until well into the 1930s, carbide lamps specially produced for this purpose were also used as bicycle lights. These carbide bicycle lamps shone far brighter than corresponding oil or candle lamps.
- The formation of ethine is used to determine the residual moisture of soil or concrete samples . Here, the sample is filled into a standardized steel bottle together with an ampoule of calcium carbide and four steel balls and sealed with a pressure gauge head. The bottle is shaken, which further crushes the sample and breaks the calcium carbide ampoule. The gas pressure resulting from the reaction of calcium carbide and moisture can be read on the pressure gauge head and converted into the moisture content. This process is known as the carbide method (CM) .
- Calcium carbide is used as an active ingredient in repellents to deter voles and moles . The gas mixture that arises on contact with the soil moisture drives the animals away without killing them.
Carbide shooting
If you put a few pieces of calcium carbide in a milk can and sprinkle them with a little water, an explosive gas mixture of ethine and oxygen arises in the volume sealed with a lid or a football . The gas mixture is then ignited through a previously drilled hole in the ground. The explosion can throw the lid or soccer ball up to 70 m. Unlike the potato cannon , the direction is less predictable. The tradition of carbide shooting is maintained primarily in the Netherlands and in some parts of northwest Germany and northern Bavaria by young men at weddings, New Year's Eve and New Year's. In Upper Lusatia it is known as Easter shooting . In the East Frisian region of Germany and the eastern parts of the Netherlands, carbide shooting is an integral part of traditional New Year's Eve celebrations. In Carinthia (Austria) carbide shooting takes place as an Easter custom from the food blessing on Holy Saturday to the resurrection celebration on Easter Sunday in the morning.
Carbide fishing
The same principle was used by poor people in the starvation period after the Second World War , in order to be able to kill and clear many fish quickly by exploding a carbide can that was easily available at the time in fish waters , despite the ban on dynamite fishing .
Fuel gas for welding
Carbide was used extensively until around 1960 in order to generate the high-energy fuel gas acetylene (ethyne) in carbide developers at low pressure, which - in combination with oxygen - is uniquely good for welding ( gas fusion welding ) of steel ( gas fusion welding ) due to its particularly high flame temperature. Iron alloys ) are suitable for the production of pipelines in the gas / water / heating sector. Larger gas generators were permanently installed in workshops, smaller ones for mobile use on construction sites. For this, carbide had to be stored in tin cans, for example, protected from the ingress of water vapor.
This process of generating acetylene on-site was necessary because if acetylene were compressed for storage in a gas cylinder, it would decompose catastrophically while releasing heat.
It was only with the technical development of the acetylene cylinder , in which this gas can be safely stored dissolved in a solvent under medium-high pressure, that the gas supply could be converted to cylinders and simplified for the user. Acetylene bottles have long contained carcinogenic asbestos and are still heavy and sensitive to shock and heat due to solvents and porous support.
See also
Individual evidence
- ↑ a b Entry on calcium carbide. In: Römpp Online . Georg Thieme Verlag, accessed on November 10, 2014.
- ↑ a b c d data sheet calcium carbide (PDF) from Carl Roth , accessed on December 14, 2010.
- ↑ a b Entry on calcium carbide in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ Entry on Calcium acetylide in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-20.
- ↑ M. Binnewies et alii: Allgemeine und Anorganische Chemie . 2nd Edition. Spectrum, 2010, ISBN 3-8274-2533-6 , pp. 383 .
- ↑ Producing carbide with plastic waste , BINE Information Service - Projektinfo 08/2011.
- ↑ a b c Georg Brauer (ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume II, Ferdinand Enke, Stuttgart 1978, ISBN 3-432-87813-3 , pp. 931-932.
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig 1985, ISBN 3-342-00280-8 , p. 263.
- ↑ http://www.csb.gov/csb-issues-safety-bulletin-releases-findings-and-recommendations-in-2005-acetylene-services-company-asco-explosion-that-killed-three-in-perth -amboy-nj /
- ↑ Vietmeier, Andreas: Wühlmäuse ( water voles) in the garden. In: www.landwirtschaftskammer.de . Agriculture Chamber of North Rhine-Westphalia , Plant Protection Service, accessed on March 19, 2016.
- ↑ Approval report for Prontox vole gas. Federal Office for Consumer Protection and Food Safety, accessed on March 19, 2016.
- ↑ "The device" lets it rip properly. Retrieved December 29, 2019 .
Web links
- German Chemistry Museum: Carbide production in Piesteritz in the 20th century, German Chemistry Museum Merseburg