Fatty acid synthesis
Biological fatty acid synthesis is an anabolic , assimilating metabolic process in which fatty acids are produced (e.g. for the purpose of storing energy). It proceeds through the successive cultivation of malonyl-CoA on an initially existing acetyl group that is bound to coenzyme A. In contrast to the breakdown of fatty acids, the β-oxidation , which takes place in the mitochondrial matrix, in animals and fungi they are built up in the cytosol .
Fatty acid synthesis in plants
In plants, fatty acid synthesis takes place only in the plastids , in green plant cells in the chloroplasts , otherwise in the chromoplasts , leucoplasts or proplastids . The same reactions take place as in the cytosol, but only fatty acids up to C18 are synthesized. These can obtain a maximum of one double bond through a soluble stroma desaturase . After transport into the smooth endoplasmic reticulum (sER), the chain is lengthened; further double bonds can be incorporated in the sER by means of membrane-bound desaturases. In plants, fatty acids are not broken down in the mitochondria, but only in peroxisomes .
Long-chain fatty acids are the starting point for the biosynthesis of wax and cutin in plants , both of which are indispensable for protection from the environment.
General reaction process
For the exact biosynthesis including structural formulas see section web links
It is noteworthy that the fatty acid under construction in mammals and fungi remains bound to a multifunctional enzyme , the so-called fatty acid synthase , which carries all seven enzyme functions, until it is finally completed . It has a peripheral (distal) SH group and a central (proximal) SH group on a subunit of the complex, the acyl carrier protein domain (ACP). In plants and bacteria, the individual enzyme functions and ACP are distributed among different proteins that assemble to form a protein complex . In all eukaryotes there is also a second fatty acid synthesis pathway in the mitochondria , in which the enzyme activities are distributed over individual proteins.
The reaction path is as follows:
- 1) Acetyl-CoA is produced in several processes, mainly through the oxidative decarboxylation of pyruvate in glycolysis , through the breakdown of amino acids or through the β-oxidation of fatty acids, the opposite of the process shown here.
-
2)

- Carboxylation (addition of CO 2 ) of free acetyl-CoA to malonyl-CoA outside the complex by acetyl-CoA carboxylase (with the prosthetic group biotin ).
- 3)
- Binding of the malonyl-CoA to the acyl carrier protein domain by the malonyl transferase , during which coenzyme A is split off again.
-
4)

- Acetyl residue condenses on the malonyl residue with the elimination of CO 2 with the help of ketoacyl synthase, an acetoacetyl group (C 4 ) is formed on the proximal SH group; with each of the eight “cycles” that the reaction goes through, this intermediate product gets more carbon atoms (hence it has a different name). Another enzyme that uses acetyl-CoA directly as a substrate can also extend the chain in plants and bacteria (KAS III, EC 2.3.1.180 ).
-
5)
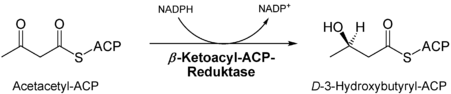
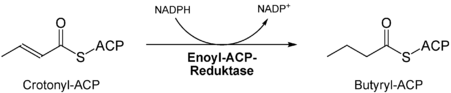
- Reduction of the keto group of the ketoacyl residue at C3 by ketoacyl-ACP reductase , the product is a hydroxyacyl residue .
-
6)

- Dehydration of the hydroxyacyl residue by the hydroxyacyl-ACP dehydratase between C2 and C3.
- 8) Addition of malonyl-CoA to the ACP domain (= step 3), then the cycle is repeated six times and thus the acyl residue on the proximal SH group is extended.
- 9) Palmitic acid : C 16 is released by an acyl hydrolase and immediately dissociates to palmitate.
This process is repeated until completion, with palmitic acid usually being hydrolytically split off by a thiolase. It should be noted that the fatty acid is initially free. The key enzyme in fatty acid synthesis is acetyl-CoA carboxylase , which is regulated both allosterically and hormonally .
The chain elongation of the fatty acids is catalyzed in the plant by elongases .
This metabolic pathway plays a minor role in humans under normal nutritional conditions, since sufficient fats are already absorbed through food. As a result, there is no need to build fatty acids from carbohydrates. In animals, the fatty acid synthesis has an even bigger role, since this z. B. need to build up a substantial reserve of fat for the winter.
Odd-numbered fatty acids
Fatty acid chains with an odd number of carbon atoms are formed when propionyl-CoA is used as the starter molecule instead of acetyl-CoA. As already described for the even-numbered fatty acids, chain lengthening then takes place through successive reactions with malonyl-CoA.
Branched chain fatty acids
Branches at the alkyl end of the fatty acid result when the synthesis begins with a branched molecule. If there is an additional methyl group on the penultimate carbon atom of the main chain , one speaks of an iso fatty acid and the penultimate one of an anteiso fatty acid. Iso- fatty acids are formed when the synthesis starts with isovaleryl-CoA or isobutyryl-CoA. These compounds are derived from the branched-chain amino acids leucine and valine . On the other hand, starting the synthesis with 2-methylbutyryl-CoA, which is derived from isoleucine , creates an anteiso fatty acid. However, methyl branches can also be generated further within the fatty acid chain during the synthesis. This happens when the chain is not extended with malonyl-CoA but with methylmalonyl-CoA .
The synthesis of branched-chain fatty acids is of great importance for a number of types of bacteria, such as mycobacteria , since branching chains are a way of regulating membrane fluidity . The biosynthesis of the longest natural fatty acids, mycolic acids , is so extensive that it cannot be presented in detail here.
See also
swell
- ↑ EC 2.3.1.85 (fatty acid synthase (MEC)).
- ↑ JK Hiltunen u. a .: Mitochondrial fatty acid synthesis and respiration. In: Biochimica et biophysica acta Volume 1797, Numbers 6-7, Jun-Jul 2010, pp. 1195-1202. doi : 10.1016 / j.bbabio.2010.03.006 . PMID 20226757 . (Review).
- ↑ EC 2.3.1.39 (ACP-S-malonyl transferase).
- ↑ EC 2.3.1.41 (β-ketoacyl-ACP synthase I).
- ↑ EC 1.1.1.100 (3-ketoacyl-ACP reductase).
- ↑ EC 4.2.1.61 (3-hydroxypalmitoyl-ACP hydratase).
- ↑ EC 1.3.1.10 (enoyl-ACP reductase).
- ↑ a b Klaus Urich: Comparative Animal Biochemistry . Springer, Berlin 1994, ISBN 3-540-57420-4 , pp. 564 f .
- ↑ T. Kaneda: Iso- and anteiso-fatty acids in bacteria: biosynthesis, function, and taxonomic significance. In: Microbiol. Rev. 55 (2); June 1991: pp. 288-302, PMID 1886522 (free full text access ).
literature
- Florian Horn: Human biochemistry . The textbook for medical studies. 3. Edition. Thieme, 2005, ISBN 3-13-130883-4 .
- Jeremy M. Berg , John L. Tymoczko , Lubert Stryer : Biochemistry . 5th edition. Spectrum Akademischer Verlag, Heidelberg 2003, ISBN 3-8274-1303-6 .
- Peter Sitte , Elmar Weiler , Joachim W. Kadereit , Andreas Bresinsky , Christian Körner : Textbook of botany for universities . Founded by Eduard Strasburger . 35th edition. Spektrum Akademischer Verlag, Heidelberg 2002, ISBN 3-8274-1010-X .
Web links
- Animation of the reaction process
- Gopinathrao / reactome.org: Fatty Acyl-CoA Biosynthesis
![{\ displaystyle \ mathrm {Malonyl {\ text {-}} CoA + ACP {\ text {-}} SH {\ xrightarrow [{ACP-S-Malonyltransferase}] {}} CoA {\ text {-}} SH + Malonyl {\ text {-}} ACP}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/015a3db00acbd781c6bcb19abb8dddb0abffbbad)