Lithium-ion capacitor
Lithium -ion capacitors ( English lithium ion capacitor , LIC) are supercapacitors with asymmetric, d. H. differently structured electrodes . They belong to the group of so-called hybrid capacitors. Their electrical capacity is made up of the series connection of a positive electrode of a conventional double-layer capacitor with a static double-layer capacitance and a secondnegative electrodefrom an accumulator and doped with lithium ionswith an additional, very high electrochemical pseudocapacitance .
The doping of the negative electrode causes the dielectric strength of the capacitor to be around 3.8 V. Since the stored energy of a capacitor grows quadratically with the voltage, the energy density (storage capacity) of lithium-ion capacitors at around 3.8 V is significantly higher than that of conventional double-layer capacitors at 2.5 V. At the same time, lithium-ion capacitors keep them The very high power density of double-layer capacitors, so they have the ability to charge and discharge quickly, coupled with a high cycle stability and long service life, which gives them clear advantages over lithium-ion batteries .
Their ability to charge and discharge quickly, coupled with a comparatively high energy density, makes lithium-ion capacitors attractive for use in new electromobility concepts , for example as a storage device for the recovery of braking energy ( recuperation ) and as an energy supplier for peak loads in trains and buses and in motor vehicles.
| Family of super capacitors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Basic structure, storage principles and family assignment
Basic structure
In terms of their basic construction, lithium-ion capacitors are similar to double-layer capacitors. They also consist of two large electrodes that are electrically connected to one another with a conductive electrolyte , an ion conductor . The electrodes are separated by an electrically permeable membrane (separator) and protected against direct contact with one another and thus against a short circuit . Flat current conductors (collectors) contact the respective electrode and connect it to the external connections. These lower parts can be wound into a roll or processed in several layers to form a stack. They are then installed in a common housing (cell) and more or less hermetically sealed.
Lithium-ion capacitors differ in one respect from conventional double-layer capacitors, which have two symmetrical, static double-layer electrodes. They combine a double-layer electrode with a battery-like electrode and thus become hybrid capacitors. The lithium-ion capacitors differ from the lithium-ion accumulators in the structure of the positive electrode, which is an electrochemical metal-oxide electrode in the Li-ion accumulators.
As hybrid capacitors, the lithium-ion capacitors have two different electrodes that have different capabilities. The positive electrode of a LIC usually consists of activated carbon , and thus corresponds to a conventional electrode of a double layer capacitor, the electric energy in an electric static Helmholtz - bilayer stores.
The special feature of the lithium-ion capacitor is the negative electrochemical redox electrode with intercalated lithium ions. Depending on the manufacturer, it consists either of specially suitable activated carbon or graphite , of a conductive polymer or of graphene in the form of carbon nanotubes and stores the electrical energy electrochemically in a so-called pseudocapacity via redox reactions combined with Faraday charge transfer transitions . Both types of storage, the static and the Faraday, have a linear relationship between the stored electrical energy and the voltage on the capacitor.
Static and electrochemical storage principle
Static double layer capacitance

The physical effect that occurs in a Helmholtz double layer of an electrode has the effect that when a voltage is applied both in the surface area of an electrode and in the electrolyte , an electrically separating layer, a double layer, is created, the mirror-image symmetrical also on the second electrode Can be found again. The “thickness” of a layer in the electrolyte is in the range of the diameter of a solvent molecule , i.e. about 0.1 to 10 nm. The anionic or cationic charges accumulate at these boundary layers when the capacitor is charged, in a voltage-dependent, mirror-image manner with an adsorption reaction . The charges of the adsorbed ions in the electrolyte are balanced by counter charges in the electrodes.
Between the accumulated charges, the ions in the electrolyte and the ions in the electrode within the phase boundaries, there is a charge separation with the formation of an electric field , the strength of which corresponds to the voltage applied. This creates a static capacitor through the Helmholtz double layer. When discharging, the ions redistribute themselves in the electrolyte after a desorption reaction .
Electrochemical pseudocapacitance
The storage of electrical energy in a pseudocapacitance takes place with the help of a simple reversible redox reaction (reduction-oxidation reaction) between the electrode and the cations in the electrolyte, which takes place on the surface of the electrode. When discharging, the cations at the negative electrode (cathode) each give off an electron, which flows via the external circuit to the positive electrode (anode). At the same time, the same number of anions migrate through the electrolyte from the negative to the positive electrode. At the positive electrode, however, it is not the ions that take up the electron again, but rather the transition metal ions that are present there and are strongly ionized in the charged state and are therefore quite “electron-hungry”.
The redox reactions are effective within narrow voltage limits like a capacitance and can also be measured in this way, although in contrast to accumulators there is no change in substance at the electrodes. The ability of electrodes to bring about redox reactions that lead to pseudocapacitance depends on the material of the electrodes. Electrodes made of special activated carbons, conductive polymers or certain metals or metal oxides, which are introduced into the electrode material by doping and with the help of an intercalation, are suitable for this, because they enable the electrochemical redox reactions. H. Storage of foreign atoms or compounds in the spaces between layers, e.g. B. of graphite inserted, where the redox reactions then take place with the appropriate cations. With the same volume or weight, a pseudo-capacitance can form a capacitance up to 100 times greater than a static capacitance in a Helmholtz double layer. That depends on the size of the atoms involved, which are usually much smaller than the ions in the electrolyte. In addition, the electrochemical redox reactions are very fast. Lithium-ion capacitors thus have a significant advantage over accumulators: the charging and discharging process is significantly faster than with accumulators.
Family assignment
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
By combining two different types of storage of electrical energy in one component, an electrostatic capacitance on the Helmholtz double layer and an electrochemical redox pseudocapacitance, it is necessary to create a system of the resulting components. This family assignment leads to a family tree that separates them into pure double-layer capacitors, into pseudo capacitors and into capacitors that have both effects, the hybrid capacitors under the umbrella term "super capacitors". This subdivision is based on the construction of the respective electrodes, the properties of which determine the function of the capacitors. In industry, supercapacitors are also often called ultracapacitors.
Functionality and properties
functionality
- How lithium-ion capacitors work
Lithium-ion capacitors (LIC) belong to the supercapacitor family and are hybrid capacitors with asymmetrical electrodes. They combine a static double-layer electrode with an electrochemical redox electrode that is doped with intercalated lithium ions and both of which are electrically connected to one another by an electrolyte containing lithium.
When a voltage is applied to the capacitor , the charge carriers in the electrolyte dissociate into their positive ( cations ) and negative charges ( anions ). At the positive electrode of a LIC, which usually consists of activated carbon , the anionic charges accumulate on the double layer, depending on the voltage, with an adsorption reaction when the capacitor is charged . This forms an internal capacitor at the positive electrode of the LIC with the capacitance that results from the surface of the electrode and the distance given by the double layer.
The special feature of the lithium-ion capacitor is the negative electrochemical redox electrode. Depending on the manufacturer, it consists either of activated carbon or graphite , of a conductive polymer or of graphene in the form of carbon nanotubes . In the negative electrode of the lithium-ion capacitor, positively charged lithium atoms are incorporated (intercalated) into the spaces between layers of the electrode material during the manufacturing process . There they transfer a charge in the form of an electron to the material of the electrode, creating an excess of electrons and lowering the potential of this electrode. This effect has the same effect as if a galvanic element had been built into the negative electrode, which generates a bias voltage (terminal voltage) of around 2.2 to 3 V. The lithium-ion capacitor is only charged when the voltage applied to the capacitor is greater than the terminal voltage. Then the positive lithium ions distributed in the electrolyte migrate to the negative electrode, where they can also form an intercalation compound and release their charge with a redox reaction. The negatively charged electrode thus forms an internal capacitor, the capacity of which results from the pseudo capacity of the intercalated lithium ions.
Since both the electrostatic energy storage on the double layer and the redox reactions in the pseudocapacitance are linear to the stored charge in the capacitor, the voltage curve on the capacitor corresponds to the stored energy. This distinguishes the capacitors from the accumulators, whose voltage at the connections remains largely constant regardless of the state of charge.
The negatively and positively charged electrodes thus form two internal capacitors, the capacities of which are connected in series via the electrolyte. The total capacitance of the capacitor results from:
with the capacitance of the doped electrode, the capacitance of the double layer electrode .
Due to the very small size of the lithium ions, a very high charge concentration arises on the doped negative electrode, with the result that the pseudocapacitance of this electrode becomes very large, much greater than the double-layer capacitance of the positive electrode. The value of the pseudocapacitance of this electrode is often greater than that of the static double-layer capacitance by a power of ten.
If, however, in a series connection of two capacitors, the value of is very much larger than the value of , then the smaller capacitance value determines the total capacitance of the capacitor.
The total capacity of a lithium-ion capacitor thus corresponds to the (static) capacity of the positive double-layer electrode.
As described above, the doping of the anode causes the potential to drop by about 2.5 V on average. The double layer of the cathode has a dielectric strength of about 1.3 V, so that the total dielectric strength of the lithium-ion capacitor is 3 .8V can be specified. Because the energy stored in a capacitor increases with the square of the voltage
significantly more electrical energy can be stored in a lithium-ion capacitor due to the increased dielectric strength of 3.8 V than in a conventional EDLC with only 2.5 V dielectric strength.
However, the lowering of the potential produced by doping the negative electrode must not be exploited when the capacitor is in operation. This means that the “effective galvanic element” in the anode is not suitable for emitting electrical energy by means of chemical processes such as in a lithium-ion battery. The LIC must therefore not be discharged to 0 V or short-circuited at the connections. Due to the structure of the lithium-ion capacitors as hybrid capacitors with a Li-doped negative electrode, lithium-ion capacitors are also polarized capacitors that may only be operated with the correct polarity.
properties
Lithium-ion capacitors are only a few standard types on the market, the technical descriptions of which are in some cases still very incomplete. Nevertheless, properties of the LICs can be filtered out from the available data, with which they can be compared with other components.
| Characteristic values | Electrolytic capacitors |
Double layer capacitors |
Super, ultra capacitors |
Lithium- ion capacitors |
Lithium- ion accumulators |
|---|---|---|---|---|---|
| Operating temperature range in ° C |
−40 ... + 125 | −20 ... +70 | −20 ... +70 | −20 ... +70 | −20 ... +60 |
| Nominal voltage per cell in V |
4… 550 | 1,2… 4 | 2.5… 4 | 2.2 ... 3.8 | 2.5 ... 4.2 |
| Charge / discharge cycle life | unlimited | 10 5 … 10 6 | 10 5 … 5 × 10 5 | 10 4 … 5 × 10 5 | 0.5… 4 × 10 3 |
| Capacity range in F | ≤ 1 | 0.1 ... 100 | 100 ... 6500 | 300 ... 2200 | - |
| Capacity per volume in F / cm 3 | 0.001 | 5… 8 | 8… 10 | 10 ... 18 | 1000 |
| Energy density in Wh / kg | - | 1.5 ... 3.9 | 4… 9 | 10… 15 | 95… 190 |
| Effective power density (kW / kg) | > 100 | 2… 10 | 3… 10 | 3… 6 | 0.3 ... 1.5 |
| Self-discharge (self-discharge time at room temperature) |
high (days) |
medium (weeks) |
medium (weeks) |
low (months) |
low (months) |
| Efficiency in% | 99 | 95 | 95 | 90 | 90 |
| Lifetime at room temperature in a | > 20 | 5… 10 | 5… 10 | 5… 10 | 3… 5 |
With the dielectric strength of lithium-ion capacitors of around 3.8 V, the energy density (storage capacity) is significantly higher than that of conventional double-layer and supercapacitors with 2.5 V, but significantly lower than that of lithium-ion batteries . On the other hand , lithium-ion capacitors retain the very high power density of double-layer capacitors, i.e. they have the ability to charge and discharge quickly, with a high degree of cycle stability, typical values go up to 1 million cycles. This high cycle stability combined with a larger temperature range means that LICs have a significantly longer service life than Li batteries. Lifetime times of 12 years at 30 ° C with a change in capacitance of only 15% are definitely achievable and thus the LI capacitors offer industry and consumers an important argument for use in self-sufficient, network-independent systems or for the recuperation of braking energy. LICs also offer advantages over LI batteries in terms of the efficiency of storing electrical energy and the self-discharge rate (<5% at 25 ° C for 3 months of storage). Lithium-ion capacitors are also classified as "environmentally friendly" because they do not use any prohibited heavy metals.
construction
- Types of lithium-ion capacitors
Basically, all lithium-ion capacitors consist of two different electrodes, which are separated from one another by a separator and which are connected to the connections to the outside world via collectors and which are installed together in a housing that is as tight as possible. The inner construction can be layered or made as a wrap. The layering or the winding comprises not only a separator layer for mechanical separation of the electrodes, but also one or two further separator layers to protect the cell from direct metallic contact with the metal housing. This structure of a lithium-ion capacitor can basically only be loaded with the maximum cell voltage. Higher dielectric strengths are achieved by connecting several capacitors in series.
ingredients
Lithium-ion capacitors do not contain any heavy metals such as cadmium, lead and mercury. They are therefore classified as environmentally friendly.
Lithium-ion capacitors differ significantly from lithium-ion accumulators in terms of the amount of lithium processed. Although a large number of lithium atoms are built into the negative electrode, the total amount of lithium in the capacitor is relatively small. For example, a 2000 F capacitor with a total weight of around 200 g only has a total mass of 0.3 g lithium. With this low proportion of lithium, there are no legal restrictions with regard to possible hazards, fire hazards or the risk of explosion with lithium-ion capacitors.
Electrodes
As hybrid capacitors, lithium-ion capacitors have two different electrodes with different properties. For the positive electrode, it should have particularly good properties for the formation of a static double-layer capacitance. For the negative electrode, it is said to have a great ability to intercalate ions to generate a large pseudocapacitance.
Electrodes for capacitors should initially have the best possible conductivity . In addition, they should have the largest possible surface area with the smallest possible volume and weight for the static double-layer capacitance. This requirement is met by electrodes made of activated carbon , graphite or graphene .
Activated carbon, graphite or graphene mainly consists of carbon and has an extremely large surface. It is up to 2000 m² / g. Carbon is electrically very conductive along the crystal planes and is therefore well suited as an electrode material.
In the simplest form, these carbon electrodes made of pressed activated powder with a highly porous structure are used. The pores are connected to each other like a sponge (open-pored) and together form the very large inner surface. For an electrode made of activated carbon with 1000 m² / g, with a typical double-layer capacitance of 10 µF / cm², a specific capacitance of 100 F / g results. In another form, activated carbon can be spun into activated carbon fibers (ACF), which can be processed into fabrics for flexible electrodes. The surface of such fabrics is usually larger than that of the sponge-like powder and can reach around 1500 m² / g. Electrodes made of activated carbon or graphite are quite inexpensive, non-toxic and do not contain any substances that are harmful to the environment. You can also use inexpensive, natural raw materials, such as. B. coconut shells, sugar or algae can be produced.
The negative electrode of the lithium-ion capacitors must be made of a material specially suitable for generating a pseudocapacitance. To do this, it must be able to intercalate ions. This “incorporation” of lithium atoms takes place both as part of the manufacturing process through doping and during operation when the capacitor is charged.
The materials already described above, activated carbon, graphite and graphene, can also have a large pseudocapacity if the pore size in the material has a very small diameter. The ability of activated carbon to store foreign atoms such as lithium in the intermediate layers of its crystal planes increases significantly when the activated carbon is covered with a conductive polymer.
Electrodes made of a conductive polymer such as polypyrrole , polyaniline , polythiophene or pentacene , a polyacene (PAS, polyacenic semiconductor ), are also quite suitable for intercalation . They are inexpensive to manufacture and have a large charge carrier mobility of up to 5 cm² / Vs. The material is amorphous in a very porous structure. This structure allows a large number of lithium ions to be stored with great stability. Li-doped PAS has the same volumetric energy density as metallic lithium, but electrodes made from a conductive polymer have a shorter service life and reduced cycle stability compared to activated carbon electrodes due to chemical instabilities in their electrochemical reactions. Compared to Li-Ion batteries, however, the service life and cycle stability of the LIC are still much longer.
More recent developments use electrodes in the form of carbon nanotubes or graphene. Graphene has a very large surface area, one gram of which has a surface area of 2675 square meters. Researchers at MIT developed electrodes for ultracapacitors with mats made of carbon nanotubes, which have a diameter of 0.7 to 2 nm with a length of several tens of µm and which can achieve a theoretical capacity of 550 F / g. J. Schindall shows this electrode quite clearly when it is loaded with ions. The two-dimensional structure of the graphene layer also improves the charging and discharging speed of a capacitor manufactured with it. The charge carriers in vertically oriented graphene nanosheets can migrate into and out of the deeper structures of the electrode more quickly and thus accelerate the switching speed.
electrolyte
The electrolyte in lithium-ion capacitors is the electrically conductive connection between the two electrodes in the capacitor. It should hold ready the anions required for the double-layer capacitance and the cations for the redox reactions of the pseudocapacitance when charging the capacitor . Its properties determine the voltage window in which the capacitor can be operated, its temperature range, the internal resistance (ESR) and, via its stability, also the long-term behavior of the capacitor.
An electrolyte always consists of a solvent in which conductive salts are dissolved. The salts dissociate in the solvent to form positive cations and negative anions and make the electrolyte conductive. The electrolyte must be able to penetrate the porous, sponge-like or cross-linked structure of the electrodes; its viscosity must be small enough to fully wet the electrode surface. It must also be chemically inert and must not chemically attack the materials of the capacitor. The electrolyte used in lithium-ion capacitors is usually anhydrous and consists of organic aprotic solvents such as ethylene carbonate , propylene carbonate , dimethyl carbonate , diethyl carbonate or 1,2-dimethoxyethane and dissolved lithium salts such as LiPF 6 or triazolates. Electrolytes with organic solvents are more expensive than aqueous electrolytes but have a higher dissociation voltage of up to about 4 V and a larger temperature range. Their somewhat lower conductivity compared to aqueous electrolytes results in a lower power density, but since the energy density increases with the square of the voltage, LICs with organic solvent electrolytes have a higher energy density than those with aqueous electrolytes. The special feature of the electrolytes for lithium-ion capacitors is that the cations for intercalation into the negative electrode must be very small. The lithium salt consequently dissociates in such a way that lithium appears in the electrolyte in the form of individual atoms. Only then can the required Faraday charge-transfer transitions of the pseudocapacitance take place.
Separators
Separators should mechanically separate the two electrodes from one another in order to prevent a short circuit. They can be very thin (a few hundredths of a millimeter) and must be very porous in order to contribute as little as possible to the internal resistance (ESR) of the capacitor. In addition, they have to be chemically inert in order to keep the influence on the long-term stability and conductivity of the electrolyte low. Inexpensive lithium-ion capacitors use open capacitor papers as separators, professional LICs use porous plastic films, glass fiber fabrics or porous ceramic fabrics as separators.
Collectors and housings
The collectors (current collectors) are used to make electrical contact with the electrode material and connect them to the terminals of the capacitor. They must have good conductivity, after all peak currents of up to 100 A should be distributed to the capacitor cell or taken from it without any problems. If the housing is made of metal as usual, the collectors and housing should be made of the same material, usually aluminum, because otherwise a galvanic cell would form in the presence of an electrolyte, which could lead to corrosion. The collectors are either sprayed onto the electrodes using a spray method or consist of a metal foil on which the electrode is attached.
Electrical characteristics
capacity
The value of the capacitance that can be measured from the outside at the connections of a lithium-ion capacitor results from the energy content of a capacitor charged with the charging voltage :
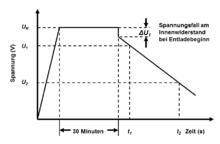
This capacity is also called "DC voltage capacity". It is measured in accordance with the applicable standard (DIN EN 62391-1) by first charging the capacitor to its nominal voltage with a constant current source. The capacitor is then held at this voltage value for 30 minutes and then discharged with a defined discharge current , the time which elapses is then determined in which the voltage drops from 80% to 40% of the nominal voltage. The capacity then results from the definition en in the adjacent figure from the formula:
The measurement methods specified by the individual manufacturers can differ in some details from the standardized method, see for example:
The standardized but very time-consuming measurement method for measuring the capacity can be calculated from the energy content and by measuring the voltage drop from 90% to 70% of the nominal voltage value using the following formula
- :
The capacity of a lithium-ion capacitor is very dependent on frequency. At a measuring frequency of 10 Hz, the measured value drops to only around 20% of the DC voltage value. This behavior is related to the limited mobility of the ions in the electrolyte, especially in the porous structure of the electrodes. The properties that result from this can be described electrically quite well with a series connection of RC elements connected in series. In order to utilize the entire capacity of a pore up to the end of the pore, all individual capacities must be achieved via the serial RC time constants, while the flowing current must overcome an ever increasing line resistance. This means that the entire capacity of the capacitor is only reached after longer switch-on times. When applying an alternating voltage, even with a very low frequency, only the greatly reduced capacity at the pore entrance is used. The frequency dependence of the capacitance also has an impact on the operation of the capacitors. If the LICs are to be operated with fast charging and discharging cycles, the full value of the DC voltage capacity is no longer available to the application. The usable capacitance value is smaller and must be adapted in each individual case to the application by selecting the appropriate capacitor.
Dielectric strength and polarity
The dielectric strength of lithium-ion capacitors is the sum of the dielectric strengths of the two electrodes. The doping with lithium creates a voltage of around 2.2 V in the negative electrode when it is connected to ground. Together with the positive double-layer electrode in series, which can be loaded with around 1.3 V, and a small additional electrode The voltage drop of the negative electrode of about 0.5 V results in the total dielectric strength of a lithium-ion capacitor of about 3.8 V. The lower potential difference between the uncharged and charged state of the two electrodes can be explained by the fact that the capacity of the negative Electrodes is much larger than that of the positive electrode and therefore the influence of the charge on the voltage is less. Depending on the manufacturer, the value of the total dielectric strength can be exceeded or not reached. If a higher voltage than the value specified by the respective manufacturer is applied to the capacitor and the maximum dissociation voltage at which the Helmholtz effect remains stable in the double layer is exceeded, the electrolyte will undergo electrolytic decomposition. This can lead to chemical reactions that lead to gas formation and thus destroy the capacitor.
Lithium-ion capacitors are polarized capacitors. The doping of the negative electrode can be destroyed if the capacitor is connected to ground with its positive pole, is short-circuited, is operated with AC voltage or with the wrong polarity. A lower voltage limit of around 2.2 V must not be exceeded for lithium-ion capacitors.
Internal resistance
The charging or discharging of a double-layer capacitor is associated with a polarization of the ions in the electrolyte and a movement of the charge carriers through the separator deep into the pores of the electrodes. This movement of the ions in the electrolyte causes losses that can be measured as the internal resistance of the capacitor. With the electrical model of serially connected RC elements in the pores of the electrodes in the picture above, it can easily be explained that the internal resistance of double-layer capacitors increases with a time delay as the penetration depth of the charge carriers into the pores of the electrodes increases. Since the charge carrier mobility is also limited, not only the capacitance but also the internal resistance is strongly frequency-dependent. When charging or discharging a capacitor, the current flow is a direct current . The effective internal resistance , sometimes also called ESR DC , is therefore a direct current resistance . It is calculated using the voltage drop that results from the extension of the straight section of the discharge voltage at the time of the start of discharge as the intersection with the discharge curve using the following formula:
The discharge current for measuring the internal resistance is specified by the respective manufacturer for LICs. Another, faster method of measuring an internal resistance is the measurement of an alternating current resistance . This alternating current resistance is called ESR (Equivalent Series Resistance). It is measured at 1 kHz and has a significantly lower resistance value.
The internal resistance determines several properties of double-layer capacitors. On the one hand, it limits the charging and discharging speed of the capacitor. Together with the capacitance of the capacitor, the resulting time constant with
This time constant determines the time limit with which a capacitor can be charged or discharged. A 100 F capacitor with an internal resistance of 30 mΩ has e.g. B. a time constant of 0.03 * 100 = 3 s, i.e. This means that after 3 s charging with a current limited only by the internal resistance, the capacitor has reached 62.3% of the charging voltage. Since it takes about 5 to fully charge the capacitor , the voltage has reached the charging voltage after about 15 s.
The internal resistance is also the limiting factor when using lithium-ion capacitors to take advantage of the rapid charging / discharging capability compared to accumulators. Because with the very high charging and discharging currents that occur in power applications with these capacitors, internal losses occur,
which lead to a heating of the capacitor via the internal resistance . This warming is the main reason for the size limitation of the charging and discharging currents, especially when charging / discharging processes occur frequently.
Since both the charge carrier mobility of the ions in the electrolyte and the conductivity of the electrolyte compared to electrons in metallic conductors are significantly lower, the internal resistance of EDLCs is higher than with other capacitor technologies, but significantly lower than with accumulators and also shows a significantly better low-temperature behavior. However, both properties depend heavily on the composition of the electrolyte and differ significantly between the different series from the various manufacturers.
Power density and energy density
Compared to accumulators, lithium-ion capacitors can be charged or discharged much faster and thus increase the availability of the devices. This is a decisive application criterion of LICs compared to accumulators and can be found in the term power density , a power specification that is either based on its mass and is then given as gravimetric power density in kW / kg or as volume power density in kW / cm 3 . It is determined by the heat generated by the current load via the internal resistance. High power densities enable applications for the buffering of consumers ( energy storage ) that briefly require or deliver a high current (e.g. regenerative braking ).
The energy density, on the other hand, is the measure of the electrical energy that can be stored in a capacitor. It is an important parameter for comparison with accumulators and is given as gravimetric energy density in Wh / kg or kWh / kg . Sometimes the energy density is also related to the building volume, then it is given as the volumetric energy density in Wh / cm 3 or kWh / cm 3 .
Power density and energy density are usually shown in a so-called Ragone diagram . With such a diagram, the classification of a certain storage technology in comparison with other technologies can be clearly illustrated. The Ragone diagram of the energy density versus the power density clearly shows that lithium-ion capacitors have about four times the storage capacity of electrical energy compared to EDLCs, without losing the ability to quickly charge and discharge with high charging and discharging currents.
lifespan
In general, the service life of lithium-ion capacitors is heavily dependent on the purity and quality of the materials used. In addition, the service life, similar to that of double-layer capacitors , depends on the operating voltage and the operating temperature. However, this new capacitor technology is still in the start-up phase today (2011), so that further information is not yet available.
Cycle stability
The ability of capacitors to withstand low-resistance charging and discharging operations is described by the term "cycle stability". The currents that occur when charging or discharging lithium-ion capacitors can be very large. For example, to check the suitability of LICs with a capacity of 2000 F for energy recovery in buses or large industrial machines, the capacitors with 50, 100 and 150 A were tested in cyclic operation with a cycle duration of 60 s.
With such high currents, there is not only strong internal heating of the capacitors, in which the thermal expansion creates an additional stress factor, but also strong electromagnetic forces that affect the strength of the electrode-collector connection. A high cycle stability of lithium-ion capacitors with up to 500,000 cycles, whereby the change in capacitance compared to the initial value is less than ± 10%, is not only a question of the chemical stability of the components, but also the result of a mechanically robust and stable construction.
Residual current and self-discharge
In charged lithium-ion capacitors, as in all high-capacity capacitors, a so-called residual current, also known as leakage current, can occur. This residual current is temperature and voltage dependent. The higher the temperature and the higher the voltage, the greater the residual current.
The residual current is specified under the term “self-discharge”. The voltage loss is specified within a defined time. The self-discharge of lithium-ion capacitors is lower than that of standard double-layer condensates and about as large as that of accumulators and is around 5% per month at room temperature.
Comparison of parameters
Lithium-ion capacitors are only a few standard types on the market, the technical descriptions of which are in some cases still very incomplete. With this new technology, other types developed in response to customer requirements will only expand the range in a few years. The characteristic values of the lithium-ion capacitors currently (2011) offered by the various manufacturers are also a reflection of the respective state of development.
| Manufacturer type |
Maximum voltage in V |
Minimum voltage in V |
Capacity in F |
Internal resistance in mΩ |
Maximum current in A |
/ Charge discharge cycles |
Energy density in Wh / kg |
Dimensions in mm |
||
|---|---|---|---|---|---|---|---|---|---|---|
| B. | L. | H | ||||||||
| ACT Premlis |
4.0 | 2.0 | 2000 | 5.5 | 100 | 70000 | 15th | - | - | - |
| FDK EneCapTen |
4.0 | - | 2000 | 1.5 | - | 500000 | 14th | 100 | 110 | 13 |
| JM Energy Ultimo |
3.8 | 2.2 | 2200 | 1.5 | 100 | - | 11 ... 14 | 138 | 106 | 10.5 |
| NEC Tokin LIC |
3.8 | 2.2 | 1100 | 1.8 | - | 10,000 | 14th | 192 | 95 | 5.5 |
| Taiyo Yuden LIC |
3.8 | 2.2 | 200 | 50 | 2… 10 | 100,000 | 10 | 25th | 25th | 40 |
Note on marking the polarity
Lithium-ion capacitors store electrical energy using an electrochemical process. They are thus similar in their mode of operation to the accumulators . When the electrodes are identified by the terms anode and cathode , there can be confusion, depending on whether a component is viewed as a producer or a consumer. Because in an electrical generator for direct voltage (accumulator) the cathode has positive polarity (+). In contrast, in an electrical consumer - capacitors are consumers - the cathode has negative polarity (-). In the following, the electrodes are therefore only named with their polarity.
Advantages and disadvantages
Advantages and disadvantages compared to LI batteries
The hybrid construction of lithium-ion capacitors with a negative rechargeable battery electrode doped with lithium-ion and a positive lithium-free double-layer electrode made of activated carbon offers some fundamental advantages over lithium-ion rechargeable batteries .
- The risk of fire is significantly lower. Because in lithium-ion accumulators, overcharging or overloading of the positive electrode can lead to chemical reactions with a fire hazard if lithium metal oxides of the spinel type react with the electrolyte. Since lithium-ion capacitors have a lithium-free double-layer electrode, no chemical reactions can take place with the electrolyte. You may even survive a “nail test”.
- They also have a significantly lower requirement for lithium
- and only use environmentally friendly materials, prohibited heavy metals are not used
With the LI capacitors, the electrical energy is stored by two physical processes, static in the double layer and faradaysch in the pseudocapacity and not by a chemical process as with Li-ion batteries. This gives LICs the following advantages:
- LICs cannot be overcharged if the applied voltage remains in the nominal voltage range.
- They have a significantly higher power density with the ability to charge and discharge quickly
- LICs are cycle-proof and they have a significantly higher number of charge / discharge cycles of at least 500,000 cycles within their service life
- LICs have a significantly longer lifespan (> 10 years) because the general lack of chemical processes does not affect the characteristic values.
- A smaller internal resistance, which enables very high peak currents with less self-heating with high currents, can also be explained by the type of storage without chemical processes,
- LICs have a wider temperature range, −20 to 70 ° C versus −20 to 60 ° C
- They are maintenance free
On the other hand, there are the following disadvantages compared to LI accumulators:
- The price is significantly higher
- The energy density is significantly lower, which means that an LIC stores significantly less electrical energy per volume than an LI battery
- The number of providers is (still) quite limited
Advantages and disadvantages compared to EDLCs
Lithium-ion capacitors also have advantages over double-layer capacitors:
- They have a significantly higher energy density with about four times the storage capacity based on the same construction volume
- and the higher nominal voltage of around 3.8 V facilitates the design of the electronic control by reducing switching losses.
On the other hand, there are the following disadvantages:
- a lower cycle stability with a lower number of charge / discharge cycles
- the voltage must not fall below a minimum of 2.2 V.
- the LI capacitors are also not short-circuit proof
- the price of LI capacitors is significantly higher than that of EDLCs
Applications and market
Lithium-ion capacitors as a relatively new capacitor technology are in the industrialization phase in 2010 and 2011. This means that capacitor manufacturers are preparing their production for future mass production and electronics manufacturers are developing new circuits and concepts. The capacitors are offered by several manufacturers under different names: "Premlis", ACT, "EneCapTen", FDK, "Ultimo", JM Energy, "Nano-hybrid Capacitor", NCC, "LIC", NEC-Tokin. "Lithium Ion Capacitor", Taiyo Yuden,
Lithium-ion capacitors are characterized by a higher energy density than double-layer and supercapacitors and have the same high power density, i.e. the same ability to charge and discharge quickly. For this reason, existing applications in which double-layer or supercapacitors are used will initially be replaced by new LICs, because the solution with lithium-ion capacitors either requires less space or, if the available space is used, leads to a higher energy density. The following applications should be mentioned here:
- Intermediate energy storage in wind turbines and in photovoltaic power generation ( solar systems ) with fluctuating loads
- Uninterruptible power supply systems (UPS),
- Energy storage in self-sufficient street lighting.
- Energy recovery in braking systems of industrial plants, such as forklifts and cranes,
- Energy recovery in braking systems of railways, trains and buses ( recuperation )
- Battery replacement for electric screwdrivers, fast charging possible
The market for lithium-ion capacitors is estimated at around 60 million euros with around 40 million units in 2020.
Development tendencies
Lithium-ion capacitors were developed at the beginning of the new 21st century. Global marketing began in 2005 by FDK, Asahi Chemical Industry (ACT) followed in 2006, then JMEnergy. The first of these hybrid capacitors, which combined the storage principle of double-layer capacitors with the accumulator technology of lithium-ion accumulators in one housing, worked with electrodes made of activated carbon. The negative electrode in these capacitors was doped with lithium ions, the positive electrode corresponded to that of a conventional EDLC. An aqueous solution of conductive salts was used as the electrolyte.
In order to improve the temperature behavior and make the capacitors especially suitable for use in the automotive sector, the water-containing electrolyte was replaced by an electrolyte containing lithium salts based on organic solvents in the further development of the LICs.
Another development took place in electrodes. Although the activated carbon has an extremely large surface, materials have been found whose structure has even larger surfaces. Conductive polymers such as pentacene or polythiophene have such large internal surfaces. By doping the negative electrode with lithium or lithium titanate (Li 4 Ti 5 O 12 ), very good conductivity values are achieved. Investigations at the Los Alamos National Laboratory showed that LICs with such electrodes achieve energy densities of 39 Wh / kg and power densities of 35 kW / kg.
Stimulated by the increasing pressure from the automotive sector to store electrical energy with high capacity and quick response times, a whole series of research projects are concerned with lithium-ion capacitors. The relatively new technology of being able to manufacture carbon nanotubes with great precision and larger dimensions was also examined with regard to the capacitive properties. At the University of Tokyo , Graduate School of Agriculture, nanocrystalline lithium titanate was stored in the carbon nanotubes. The resulting electrodes can achieve specific capacitance values of up to 180 F / g. Correspondingly constructed capacitors, called “nanohybrid capacitors”, achieve energy densities of 40 Wh / l and power densities of 7.5 kW / l and can thus be charged or discharged just as quickly as conventional EDLCs.
Individual evidence
- ↑ a b Dagmar Oertel: TAB, Energy Storage - Status and Perspectives. ELECTROCHEMICAL CAPACITORS, p. 86 ff. ( Online ; PDF; 1.7 MB).
- ↑ a b c H. Gualous, G. Alcicek, Y. Diab, A. Hammar, P. Venet, K. Adams, M. Akiyama, C. Marumo: ESSCAP'2008 - Lithium Ion capacitor characterization and modeling. ( Online ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. ).
- ↑ a b c d James Banas: JM Energy's Lithium Ion Capacitor: The Hybrid Energy Storage Advantage. JRS-Micro, 2009 (Lecture, slides as PDF ( memento of the original from March 6, 2016 in the Internet Archive ) Info: The archive link has been inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. ).
- ^ BE Conway: Transition from 'Supercapacitor' to 'Battery' Behavior in Electrochemical Energy Storage . In: Journal of The Electrochemical Society . tape 138 , no. 6 , May 1991, pp. 1539-1548 , doi : 10.1149 / 1.2085829 .
- ^ BE Conway: Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications . Springer, Berlin 1999, ISBN 0-306-45736-9 , pp. 1–8 ( limited preview in Google Book search). See also Brian E. Conway in Electrochemistry Encyclopedia: ELECTROCHEMICAL CAPACITORS Their Nature, Function, and Applications ( April 30, 2012 memento in the Internet Archive ) (accessed December 7, 2015)
- ↑ a b Adam Marcus Namisnyk and JG Zhu: A Survey of Electrochemical super-capacitor technology . 2003 ( PDF [accessed December 7, 2015] Bachelor thesis; University of Technology, Sydney; 2003).
- ^ AK Shukla, S. Sampath, K. Vijayamohanan: Electrochemical supercapacitors: Energy storage beyond batteries . In: Current science . tape 79 , no. 12 , 2000, pp. 1656-1661 ( PDF ).
- ↑ a b Mustapha Jammal: State of the art and application of supercapacitors . Grin-Verlag, 2009, ISBN 978-3-640-52396-2 ( limited preview in the Google book search - diploma thesis, Technical University Berlin).
- ↑ a b Marin S. Halper, James C. Ellenbogen: Supercapacitors: A Brief Overview. ( PDF )
- ↑ a b Reinhard Meyer: Energy storage between lithium-ion batteries and supercaps . November 24, 2010
- ↑ JRS-Micro, JM Energy's Lithium Ion Capacitor: The Hybrid Energy Storage Advantage, Estimated Life, page 38, PDF ( Memento of the original from March 6, 2016 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ BP Bakhmatyuk, B. Ya. Venhryn, II Grygorchak, MM Micov, SI Mudry: Intercalation Pseudo-Capacitance In Carbon Systems Of Energy Storage. ( Online ; PDF; 65 kB).
- ↑ Masayuki Morita, Masaharu Araki And Nobuko Yoshimoto: Pseudo-capacitance of Activated Carbon Fiber Coated by Polythiophenes. ( Online ; PDF; 107 kB).
- ↑ Learning unit Conductive Polymers: Polythiophene . In: ChemPedia. Fachinformationszentrum Chemie GmbH.
- ↑ a b c The use of PAS capacitors / lithium capacitors for adapting to diversification of energy supply ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. . Taiyo Yuden (company information material, PDF).
- ↑ Shizukuni Yata, Eiji Okamoto, Hisashi Satake, Hidekazu Kubota, Masanori Fujii, Tomohiro Taguchi, Hajime Kinoshita: Polyacene capacitors . In: Journal of Power Sources . tape 60 , no. 2 , May 1996, pp. 207–212 , doi : 10.1016 / S0378-7753 (96) 80012-7 ( limited preview in Google book search).
- ↑ Joel Schindall: The Charge of the Ultra - Capacitors . spectrum.ieee.org. November 2007. Retrieved July 22, 2011.
- ↑ a b Katsuhiko Naoi: 'Nanohybrid Capacitor': The Next Generation Electrochemical Capacitors . In: Fuel Cells . tape 10 , no. 5 , 2010, p. 825-833 , doi : 10.1002 / fuce.201000041 .
- ↑ Minato Egashira, Kazuteru Ueda, Nobuko Yoshimoto, Masayuki Morita: Lithium-ion Capacitor Using Lithium Triazolate as Electrolyte Salt . In: 214th ECS Meeting, MA2008-02 . Honolulu, HI 2008 ( abstract as PDF presentation).
- ↑ M. Waidhas: Basic Technology of Double Layer Capacitors. Frankfurt am Main, January 21, 2004 (Lecture, slides as PDF ( memento of the original from August 30, 2004 in the Internet Archive ) Info: The archive link has been inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. ).
- ↑ Nesscap Ultracapacitor, Technical Guide 2008. Nesscap Co., 2008 ( PDF ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this note. ).
- ↑ P. Van den Bossche, F. Van Mulders, B. Verbrugge, N. Omar, H. Culcu, J. Van Mierlo: The Cell versus the System: Standardization challenges for electricity storage devices. ( Online ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this note .; PDF; 976 kB)
- ↑ M. Bodach, H. Mehlich: Reliability aspects in the use of supercaps. IEEE Chapter Meeting Chemnitz, 11.-12. May 2006 (lecture, slides as PDF ).
- ↑ Premlis ( Memento of the original from June 16, 2011 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. . Advanced Capacitor Technology (product page).
- ^ FDK's New Product Development. Contributing to an energy-saving society by developing and providing capacitors. FDK Social & Environmental Report 2008, p. 7–8 ( PDF ( Memento of the original dated December 5, 2015 in the Internet Archive ) Info: The archive link has been inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. ).
- ↑ Introducing JM Energy Lithium Ion Capacitor, ULTIMO ( Memento of the original from October 6, 2011 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. . jm energy, 2009 (product page).
- ↑ NCC, Nano-hybrid Capacitor ( Online ; PDF; 141 kB).
- ↑ Miyagawa Risa, Hato Yukinori, Inagawa Masako, Inoue Koji. Seki Daisuke: Development of High-Power Lithium-Ion Capacitor . In: NEC technical papers . vol. 5, no. 4 , 2010, p. 52-56 ( PDF ).
- ↑ Taiyo Yuden'S New Hybrid Lithium Ion Capacitors Provide Energy Densities Up To 10 Times Greater Than Edlcs . Taiyo Yuden, October 26, 2010 (press release).
- ↑ Stanley Electric and Tamura announce: Development of “Super CaLeCS”, an environment-friendly EDLC-powered LED Street Lamp Nippon Chemi-Con, October 30, 2010 (press release, online ; PDF; 327 kB).
- ↑ Erik Sofge: Coleman's FlashCell: Yes, a Cordless Screwdriver That Really Charges in 90 Seconds . Popular Mechanics, October 1, 2009, accessed August 18, 2011.
- ↑ Six trends in lithium-ion capacitor. iceach.com, September 2, 2010, accessed August 18, 2011 .
- ^ Lithium Ion Capacitor and market trends . solar-poweronline.info. Archived from the original on March 12, 2011. Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. Retrieved July 22, 2011.
- ↑ The market of lithium ion capacitor has a bright future ( Memento of the original from August 18, 2011 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. . electronics-tech, September 3, 2010 (no longer online).
- ↑ FDK To Begin Mass Production of High-Capacity Li-Ion Capacitors . Green Car Congress, January 4, 2009.
- ↑ Masaru Yoshida: Japanese University Triples Energy Density of Electric Double Layer Capacitor . March 12, 2009 (news item).
- ↑ Nippon Chemi-Con announces: The advanced new technology Nano-hybrid Capacitor ( Memento of the original from October 25, 2010 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF; 1.1 MB). Nippon Chemi-Con Corporation, March 26, 2010 (press release).
- ↑ Katsuhiko Naoi, Shuichi Ishimoto, Yusaku Isobe, Shintaro Aoyagi: High-rate nano-crystalline Li 4 Ti 5 O 12 attached on carbon nano-fibers for hybrid supercapacitors . In: Journal of Power Sources . tape 195 , no. 18 , September 15, 2010, p. 6250-6254 , doi : 10.1016 / j.jpowsour.2009.12.104 .