Lithium aluminum hydride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Lithium aluminum hydride | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | LiAlH 4 | |||||||||||||||
| Brief description |
colorless powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 37.95 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
0.92 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
125 ° C (decomposition) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−116.3 kJ mol −1 |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Lithium aluminum hydride (LAH) is an inorganic reducing agent with the empirical formula LiAlH 4 .
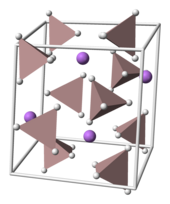
Crystal structure
Lithium aluminum hydride crystallizes in the monoclinic space group P 2 1 / c . The unit cell has the following structural parameters : a = 4.8254, b = 7.8040, and c = 7.8968 Å , α = γ = 90 ° and β = 112.268 ° (300 K). Li + -atoms each of five AlH 4 - tetrahedra surrounded.
synthesis
In the laboratory, lithium aluminum hydride is suspending of lithium hydride and aluminum chloride in diethyl ether won. After filtering off the lithium chloride and removing the ether, lithium aluminum hydride remains.
- Synthesis of lithium aluminum hydride from lithium hydride and aluminum chloride
Technically, it is also produced by reacting sodium aluminum hydride with lithium chloride. The sodium aluminum hydride required can be obtained from the elements sodium , aluminum and hydrogen at elevated temperature under pressure.
Responsiveness
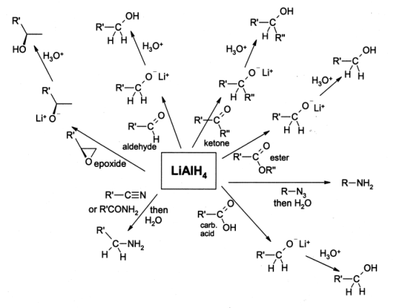
Lithium aluminum hydride is a strong reducing agent in organic-synthetic chemistry and selectively reduces almost all carbon-heteroatom double and triple bonds such as carbonyls or nitriles , but it is gentle on C = C double bonds and C DreC triple bonds ( alkenes / alkynes ), unless these are conjugated to certain activating groups; so z. B. the grouping phenyl-CH = CH-NO 2 reduced to 2-phenylethylamine . It reduces nitro compounds , amides , azides or oximes to primary amines , carbonyl compounds to alcohols , carboxylic acids , esters , acid chlorides and acid anhydrides to primary alcohols. Haloalkanes are reduced to alkanes.
It reacts violently and strongly exothermically with water to form lithium hydroxide , aluminum hydroxide and hydrogen .
Lithium aluminum hydride is metastable at room temperature . It slowly decomposes to lithium hexahydridoaluminate Li 3 AlH 6 and lithium hydride, which can be accelerated by using catalysts and heating.
Thermal decomposition takes place in three steps at higher temperatures. In the temperature range between 150 ° C and 175 ° C, the lithium hexahydridoaluminate is initially formed with the elimination of aluminum and hydrogen:
- Δ R H = 3.46 kJ mol −1
This then breaks down further into lithium hydride, aluminum and hydrogen in the temperature range between 220 ° C and 270 ° C:
- Δ R H = 14.46 kJ mol −1
The lithium hydride and aluminum formed then form a lithium-aluminum alloy in the temperature range between 585 ° C. and 606 ° C. with further release of hydrogen.
- Δ R H = 34.39 kJ mol −1
All three partial reactions are endothermic.
Lithium aluminum hydride is usually melted first, immediately followed by decomposition to Li 3 AlH 6 . At over 200 ° C, this in turn breaks down into aluminum and lithium hydride, which react at 400 ° C to form LiAl.
use
Like sodium borohydride , lithium aluminum hydride is used as a reducing agent in organic chemistry . This use as a reducing agent is an example of a synthesis method that proceeds with low atom economy . In conjunction with chiral reagents, e.g. B. TADDOL , it is possible to make enantioselective reductions of ketones .
Another application is the synthesis of sodium and potassium aluminum hydride, which can be obtained by using the corresponding hydrides .
literature
- AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 .
- Reinhard Brückner : reaction mechanisms. 3. Edition. Spektrum Akademischer Verlag, Munich 2004, ISBN 3-8274-1579-9 .
- Hans Beyer , Wolfgang Walter : Textbook of organic chemistry. 19th edition. S. Hirzel Verlag, Stuttgart 1981, ISBN 3-7776-0356-2 .
Web links
Individual evidence
- ↑ a b c Entry on lithium aluminum hydride. In: Römpp Online . Georg Thieme Verlag, accessed on May 30, 2014.
- ↑ a b c data sheet lithium aluminum hydride (PDF) from Merck , accessed on January 19, 2011.
- ↑ a b Entry on lithium alanate in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ Entry on lithium tetrahydridoaluminate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-5.
- ↑ OM Løvvik, SM Opalka, HW Brinks, BC Hauback: Crystal Structure and Thermodynamic Stability of the Lithium Alanates LiAlH 4 and Li 3 AlH 6 . In: Physical Review B . tape 69 , no. 13 , 2004, pp. 134117 , doi : 10.1103 / PhysRevB.69.134117 .
- ↑ AE Finholt, AC Bond, HI Schlesinger: lithium aluminum hydride, aluminum hydride and lithium gallium hydrides, and Some of Their Applications in Organic and Inorganic Chemistry. In: J. Am. Chem. Soc. 69, 1947, pp. 1199-1203.
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 .
- ↑ D. Seebach, H.-O. Kalinowski, W. Langer, G. Crass, E.-M. Wilka: Chiral Media for Asymmetric Solvent Inductions. (S, S) - (+) - 1,4-bis (Dimethylamino) -2,3-Dimethoxybutane from (R, R) - (+) - Diethyl Tartrate In: Organic Syntheses . 61, 1983, p. 24, doi : 10.15227 / orgsyn.061.0024 ; Coll. Vol. 7, 1990, p. 41 ( PDF ).
- ^ CH Park, HE Simmons: 1,10-Diazacyclooctadecane In: Organic Syntheses . 54, 1974, p. 88, doi : 10.15227 / orgsyn.054.0088 ; Coll. Vol. 6, 1988, p. 382 ( PDF ).
- ↑ YK Chen, S.-J. Jeon, PJ Walsh, WA Nugent: (2S) - (-) - 3-exo- (morpholino) isoborneol In: Organic Syntheses . 82, 2005, p. 87, doi : 10.15227 / orgsyn.082.0087 ( PDF ).
- ↑ JP Barnier, J. Champion, JM Conia: Cyclopropanecarboxaldehyde In: Organic Syntheses . 60, 1981, p. 25, doi : 10.15227 / orgsyn.060.0025 ; Coll. Vol. 7, 1990, p. 129 ( PDF ).
- ↑ B. Koppenhöfer, V. Schurig : (R) -Alkyloxiranes of High Enantiomeric Purity from (S) -2-Chloroalkanoic Acids via (S) -2-Chloro-1-Alkanols: (R) -Methyloxirane In: Organic Syntheses . 66, 1988, p. 160, doi : 10.15227 / orgsyn.066.0160 ; Coll. Vol. 8, 1993, p. 434 ( PDF ).
- ↑ MT Reetz, MW Drewes, R. Schwickardi: Preparation of Enantiomerically Pure α-N, N-Dibenzylamino Aldehydes: S-2- (N, N-Dibenzylamino) -3-Phenylpropanal In: Organic Syntheses . 76, 1999, p. 110, doi : 10.15227 / orgsyn.076.0110 ( PDF ).
- ^ R. Oi, KB Sharpless: 3 - [(1S) -1,2-Dihydroxyethyl] -1,5-Dihydro-3H-2,4-Benzodioxepine In: Organic Syntheses . 73, 1996, p. 1, doi : 10.15227 / orgsyn.073.0001 ; Coll. Vol. 9, 1998, p. 251 ( PDF ).
- ↑ U. Wietelmann: Applications of Lithium-Containing hydride for Energy Storage and Conversion. In: Chem. Ing. Techn. 86, 2014, pp. 2190-2194, doi: 10.1002 / cite.201400097 .
- ↑ TN Dymova, DP Aleksandrov, VN Konopolev, TA Silina, AS Sizareva: In: Russ. J Coord. Chem. 20, 1994, pp. 279-285.