Ranitidine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| ( E , Z ) -isomer mixture: ( E ) -form (top) and ( Z ) -form (bottom) | |||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Ranitidine | ||||||||||||||||||
| other names |
N - {2 - [({5 - [(Dimethylamino) methyl] furan-2-yl} methyl) sulfanyl] ethyl} - N ' -methyl-2-nitroethene-1,1-diamine |
||||||||||||||||||
| Molecular formula |
|
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class |
Ulcer Therapeutics |
||||||||||||||||||
| Mechanism of action | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | |||||||||||||||||||
| Melting point |
|
||||||||||||||||||
| solubility |
soluble in acetic acid and water, soluble in methanol (ranitidine hydrochloride) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Ranitidine is a drug selected from the group of H 2 antihistamines , for the suppression of gastric acid production at heartburn , for the treatment of gastroesophageal reflux disease and gastric ulcer - prophylaxis both in human medicine and in veterinary medicine can be applied.
In April 2020, the Food and Drug Administration (FDA) recalled all ranitidine-containing drugs from the US market, and in the same month the European Medicines Agency recommended suspending approval for such drugs in the EU . This was preceded by the finding that drugs containing ranitidine contained small amounts of N-nitrosodimethylamine (NDMA), which is classified as possibly carcinogenic, as an impurity.
Sales accrual
In Germany, ranitidine preparations require a prescription, but preparations for ingestion in strengths of up to 75 mg and pack sizes of up to 1050 mg, provided that they are used in patients over 16 years of age, in the areas of application “For heartburn and / or acid regurgitation” and a maximum therapy duration of 14 days is limited.
Extraction and presentation
The synthesis of ranitidine takes place via the production of two main precursors, the conversion of which then leads to the target molecule. The synthesis of one of the precursors begins with the parallel alkylation and amination of furfuryl alcohol to give 5-dimethylaminomethylfurfuryl alcohol , which is then reacted with cysteamine hydrochloride to give dimethylaminomethylfurfuryl thioether.
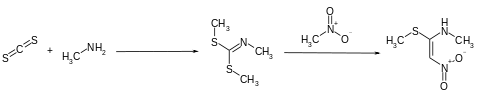
The synthesis of the second precursor begins with the conversion of carbon disulfide and methylamine in benzene using sodium hydroxide solution in the presence of tetrabutylammonium bromide to form dimethyl- N- methylcarboimidodithionate. The reaction with nitromethane then leads to N -methyl-1-methylthio-2-nitroethenamine.
The target molecule is obtained by reacting the thioether compound with the nitroethenamine.
Isomerism

Due to the interaction between the amine functions and the nitro group via a six-membered ring, the rotation barrier of the carbon-carbon double bond is only very low. Only averaged signals from ( E ) and ( Z ) isomer are found in the NMR spectrum . The system can be frozen at 271 K , where separate signals for ( E ) and ( Z ) isomer are found. At room temperature, both isomers of ranitidine are present at the same time.
effect
Ranitidine is a reversible, competitive antagonist of the histamine H 2 receptor ( H 2 antihistamine ). This receptor blockade reduces the histamine- dependent production of hydrochloric acid and the release of the digestive enzyme pepsin in the stomach . Ranitidine is about ten times stronger in this effect than cimetidine , but still has significantly fewer side effects.
Ranitidine after oral intake quickly in the intestine absorbed . It is distributed in the body, also crosses the placenta and is also excreted in the milk. Ranitidine is broken down in the liver and excreted through the kidneys . The duration of action is about 8 to 12 hours.
application
Ranitidine is used for ulcers and inflammation of the stomach, abomasum, duodenum , gastritis , esophagitis and reflux diseases. More recent guidelines, however, prefer proton pump inhibitors to H 2 blockers in the treatment of gastritis, esophagitis and reflux diseases. Ranitidine is also used, for example, to protect the stomach during cortisone therapy and occasionally as a stomach protection agent in pain therapy with non-steroidal anti-inflammatory drugs , as these often - especially in high doses and / or with long-term use - lead to stomach pain, gastric bleeding and heartburn (acid regurgitation) to lead.
When house dog ranitidine may also treat the gastrinoma (canine Zollinger-Ellison syndrome), which mastocytosis and mast cell - tumors are used. In cats Ranitidine has no significant effect on the pH of the stomach.
Contraindications and side effects
The agent must not be used in the case of known intolerance. Ranitidine should be used with caution if the kidneys are not functioning properly.
Although almost all organs have H 2 receptors, the effects on other organs are hardly detectable. Rare side effects in humans are mental confusion and headache , agranulocytosis , temporary irregular heartbeat ( bradycardia ), skin rash , nausea with vomiting , diarrhea , constipation and loss of libido .
Ranitidine should not be taken with alcohol , as ranitidine significantly increases the bioavailability of ethanol and thus leads to a significantly higher ethanol concentration. This is due to the decrease in its first-pass extraction.
Nitrosamine impurities
In September 2019, drug authorities informed about the occurrence of N-nitrosodimethylamine (NDMA) in some drug batches with the active ingredient ranitidine. This substance, classified as potentially carcinogenic, hit the headlines in 2018 in connection with the “ valsartan scandal ”. For reasons of preventive health protection, the EMA initiated recalls of all ranitidine-containing drugs that contained the active ingredient from the manufacturer Saraca Laboratories Limited on September 17, 2019. There were also indications that active ingredients from other manufacturers could also be affected. The European Medicines Agency EMA then initiated a procedure in September 2019 to assess the potential risk to patients who have taken contaminated ranitidine preparations for a long time. In April 2020, the Committee for Medicinal Products for Human Use (CHMP) recommended the EMA to suspend all authorizations of medicinal products containing ranitidine in the EU.
According to studies carried out in its own laboratory by the US mail-order pharmacy Valisure , NDMA is said to be formed by the breakdown of ranitidine under certain conditions. Such could represent the presence of air and light during storage. In particular, it should be possible for the formation of high amounts of NDMA in vivo under conditions such as exist in the human stomach or even outside the human stomach. In addition to the structural components of ranitidine ( nitro group , dimethylamino group ), the enzyme DDAH -1 could play a role. One in the US against the marketing authorization holder of ranitidinhaltigen drug Zantac raised class action corroborating the theory that the potential risks of ranitidine may be known for at least 40 years. In April 2020, the US Food and Drug Administration (FDA) recalled all prescription and non-prescription ranitidine drugs from the market. She had come to the conclusion that the concentration of the potentially carcinogenic substance in some preparations increased over time when stored above room temperature, which could lead to consumers being exposed to "unacceptable amounts" of this contamination.
Trade names
Junizac (D, excluding trade), Pylorisin (A), Ranic (A), Ranicux (D), Ranimed (CH), Raniprotect (D), Ranitic (D), Sostril (D), Ulcidin (CH), Ulsal ( A), Zantac (A), Zantic (D, CH), numerous generics (D, A, CH)
Web links
- Entry on ranitidine at Vetpharm, accessed on August 11, 2012.
Individual evidence
- ↑ a b H. N. de Armas, Os. M. Peeters, N. Blaton, E. Van Gyseghem, J. Martens, G. Van Haele, G. Van Den Mooter: Solid state characterization and crystal structure from X-ray powder diffraction of two polymorphic forms of ranitidine base. In: J Pharm Sci . 98, 2009, pp. 146-158, doi: 10.1002 / jps.21395 .
- ↑ a b c d T. J. Cholerton, JH Hunt, G. Klinkert, M. Martin-Smith: Spectroscopic studies on ranitidine - its structure and the influence of temperature and pH. In: J. Chem. Soc. Perkin Trans. 2, 1984, pp. 1761-1766, doi: 10.1039 / P29840001761 .
- ^ The Merck Index : An Encyclopedia of Chemicals, Drugs, and Biologicals. 14th edition. Merck & Co., Whitehouse Station, NJ, USA, 2006, ISBN 0-911910-00-X , pp. 1396-1397.
- ↑ Data sheet Ranitidine hydrochloride from Sigma-Aldrich , accessed on May 29, 2011 ( PDF ).
- ^ Entry on ranitidine in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ EW Gill, HR Ing: 953. Furan and tetrahydrofuran compounds analogous to ganglion-blocking agents of the 3-oxapentane-1: 5-bistrialkylammonium series. In: J. Chem. Soc. 1958, pp. 4728-4731, doi: 10.1039 / JR9580004728 .
- ↑ ARAS Deshmukh, TI Reddy, BM Bhawal, VP Shiralkar, S. Rajappa: Zeolites in organic syntheses: a novel route to functionalized ketene S, N-acetals. In: J. Chem. Soc. Perkin Trans. 1, 1990, pp. 1217-1218, doi: 10.1039 / P19900001217 .
- ↑ K. Mohanalingam, M. Nethaji, PK Das: Second harmonic generation in push-pull ethylenes: Influence of chirality and hydrogen bonding. In: J. Mol. Struct. 378, 1996, pp. 177-188, doi: 10.1016 / 0022-2860 (95) 09180-7 .
- ↑ Patent US5696275 : Process for the manufacture of pharmaceutical grade ranitidine base. Published on December 9, 1997 , Inventors: JAG MOHAN KHANNA, NARESH KUMAR, BRIJ KHERA, PURNA CHANDRA RAY.
- ^ Siegfried Hauptmann : Organic chemistry. 2nd Edition. German publishing house for basic industry, Leipzig 1985, ISBN 3-342-00280-8 , p. 516.
- ↑ S. Šutalo, M. Ruetten, S. Hartnack, CE Reusch, PH Kook: The effect of orally administered ranitidine and once-daily or twice-daily orally administered omeprazole on intragastric pH in cats. In: Journal of veterinary internal medicine / American College of Veterinary Internal Medicine. Volume 29, number 3, May-Jun 2015, pp. 840-846, doi: 10.1111 / jvim.12580 . PMID 25966746 .
- ↑ https://www.deutsche-apotheker-zeitung.de/news/artikel/2019/09/13-09-2019/nitrosaminverunireinigungen-ndma-in-ranitidin- discovered/
- ↑ https://www.deutsche-apotheker-zeitung.de/news/artikel/2019/09/17-09-2019/ranitidin-rueckruf-betapharm-hexal-1-a-pharma-abz-ratiopharm
- ↑ https://www.ema.europa.eu/en/news/ema-review-ranitidine-medicines-following-detection-ndma
- ↑ suspension of ranitidine medicines in the EU , EMA, April 30 2020th
- ↑ https://www.deutsche-apotheker-zeitung.de/news/artikel/2019/09/19-09-2019/ranitidin-bildet-tausende-von-nanogramm-ndma-im-magen
- ↑ https://www.deutsche-apotheker-zeitung.de/news/artikel/2019/09/17-09-2019/ranitidin-online-apotheke-gab-entscheidenden-nitrosamin-hinweis
- ↑ https://www.biospace.com/article/national-class-action-lawsuit-accuses-sanofi-of-concealing-zantac-cancer-risk/
- ↑ FDA Requests Removal of All Ranitidine Products (Zantac) from the Market , FDA NEWS RELEASE, April 1, 2020
- ^ A. Mende: Recall of all ranitidine preparations , Pharmazeutische Zeitung , April 2, 2020