Citric acid cycle
| Parent |
| Acetyl-CoA catabolism |
| Gene Ontology |
|---|
| QuickGO |
The citric acid cycle (also citrate cycle , citric acid cycle , tricarboxylic acid cycle , Krebs cycle or Szent-Györgyi-Krebs cycle ) is a cycle of biochemical reactions that plays an important role in the metabolism (metabolism) of aerobic cells of living beings and mainly the oxidative breakdown of organic substances for Purpose of energy production and the provision of intermediate products for biosynthesis. The acetyl-CoA , which is formed as an intermediate product when fats , sugars , alcohol and amino acids are broken down, is broken down into carbon dioxide (CO 2 ) and water (H 2 O). Intermediate products that can be used to build up organic parts of the body of the living being ( anabolism ) are formed, and energy is made available directly and indirectly in biochemically available form (as adenosine triphosphate ATP).
The citric acid cycle runs in eukaryotes in the matrix of mitochondria in prokaryotes in the cytoplasm from. A reverse sequence of reactions takes place in the so-called reductive citric acid cycle , which some bacteria use for carbon dioxide assimilation .
etymology
It is named after the intermediate product citrate , the anion of citric acid . The reaction sequence is also known as the Krebs cycle after its discoverer, Hans A. Krebs (1900–1981) . In addition to Fritz Albert Lipmann , Krebs received the Nobel Prize for Medicine in 1953 for clarifying metabolic degradation pathways.
discovery
In 1937, the biochemist Hans Adolf Krebs (in collaboration with William Arthur Johnson) was the first to postulate the citrate cycle as a way of pyruvate oxidation . Krebs examined the influence of various organic acids on oxygen consumption during pyruvate oxidation using suspensions of crushed pigeon breast muscle. This flight muscle is particularly well suited for the examination because it has a high oxidative activity due to a very high breathing speed. Krebs confirmed the observation by Albert Szent-Györgyi , among others , that C 4 - dicarboxylic acids from animal tissues ( succinate , L - malate , fumarate and oxaloacetate ) stimulate the oxygen consumption of muscles. Krebs confirmed this observation and found that pyruvate oxidation also produced such an effect. This is exemplified by C 6 tricarboxylic acids citrate, cis - aconitate and isocitrate , as well as the C 5 compound α-ketoglutarate stimulated. Other organic acids did not show the mentioned effect. However, this was extremely significant, because very small amounts already led to an oxidation of a multiple amount of pyruvate.
The second important observation of cancer was that malonate - closely related to succinate and a competitive inhibitor of succinate dehydrogenase - inhibits the aerobic utilization of pyruvate in muscle suspensions, regardless of which of the active organic acids is added. This shows that succinate and succinate dehydrogenase must be essential components of the reaction involved in pyruvate oxidation.
From these basic observations and further evidence, Krebs concluded that the active tri- and dicarboxylic acids listed below could be arranged in a chemically logical order. Since the incubation of pyruvate and oxaloacetate with minced muscle tissue caused an accumulation of citrate in the medium, Krebs concluded that this sequence does not work linearly but cyclically - its end is linked to its beginning. He was only wrong about the last missing response. The following does not apply: pyruvate + oxaloacetate → citrate + CO 2 . Thus, Krebs suggested that what he called the "citric acid cycle" was the main pathway for carbohydrate oxidation in muscle.
Role in metabolism
The degradation products of various nutrients that are broken down in the metabolism flow into the citric acid cycle. Acetyl-CoA, acetic acid bound to coenzyme A , can be described as the central breakdown product of various nutrient classes. Acetyl-CoA molecules are formed directly from fatty acids, for example, through β-oxidation . In glycolysis, carbohydrates are broken down into pyruvate (pyruvic acid), which is then decarboxylated to acetate by the pyruvate dehydrogenase complex and the acetyl residue is bound to coenzyme A. Finally, proteins are also hydrolyzed to amino acids which, after deamination , can be converted into their corresponding α- keto acids , for example α-ketoglutarate from L - glutamic acid or oxaloacetate from L -aspartate. These keto acids are often intermediates in the citric acid cycle and flow directly into it.
When acetyl-CoA is broken down via the citric acid cycle, energy is obtained in the form of GTP , as well as the reducing agents ( NADH , FADH 2 ). During these processes, the acetyl residue of the acetyl-CoA is gradually broken down into carbon dioxide and water. The electrons obtained in the citric acid cycle and bound to coenzymes (NAD + and FAD) are fed into the respiratory chain and transferred to the terminal electron acceptor oxygen (O 2 ). The energy released is used to form ATP.
The citric acid cycle also serves as a supplier of various precursor molecules for anabolism . For example, α-keto acids can be removed from the cycle in order to form amino acids or other substances.
procedure
In eukaryotes , the citric acid cycle takes place in the mitochondria , in prokaryotes in the cytoplasm or, if necessary, in mitochondrial equivalents. It is an amphibolic metabolic process, i. that is, it can serve both anabolic and catabolic pathways. The citric acid cycle is part of oxidative degradation processes and precedes the respiratory chain in aerobic organisms.
The citric acid cycle can be viewed as the third of four steps in carbohydrate catabolism. It takes place after glycolysis and oxidative decarboxylation of pyruvate to acetyl-CoA, but before end-oxidation in the respiratory chain .
The following net balance can be drawn up for the citric acid cycle:
Acetyl-CoA, acetic acid bound to coenzyme A, also known as "activated" acetic acid, is broken down into carbon dioxide (CO 2 ), hydrogen (this is bound to the hydrogen / electron carriers NADH and FADH 2 ) and coenzyme A by the citric acid cycle . Here is guanosine diphosphate (GDP) phosphorylated to guanosine triphosphate (GTP).
In the respiratory chain, the electrons bound to NADH and FADH 2 (8 reduction equivalents per acetyl-CoA ) are transferred to oxygen as the terminal electron acceptor. The energy released during the migration of electrons through the respiratory chain from protein complex to protein complex and finally to oxygen is made usable by transporting protons from the interior of the mitochondrion (matrix) into the intermembrane space and thus creating a potential difference, a chemiosmotic potential ΔP, through the proton gradient is formed. Driven by this potential, the ATP synthase finally phosphorylates ADP to ATP. Anaerobic organisms cannot let the citric acid cycle run completely; it is interrupted in them. This is because they lack the α-ketoglutarate dehydrogenase complex for converting α-ketoglutarate to succinyl-CoA or this is repressed.
When 2 pyruvate is broken down via acetyl-CoA and the citric acid cycle as well as the oxidation of the hydrogen split off (20 reduction equivalents) in the respiratory chain, 25 ATP provides significantly more energy than in the glycolysis of glucose up to 2 pyruvate, in which only 2 ATP are formed.
Partial reactions

The reaction sequence is sketched in the figure on the right. The starting point of the citric acid cycle is a condensation 1 of oxaloacetate with acetyl-CoA catalyzed by citrate synthase to form citrate. If necessary, citrate is withdrawn from the cycle and fed into cholesterol biosynthesis or fatty acid synthesis . These processes taking place in the cytosol require acetyl-CoA, which - in contrast to citrate - is unable to cross the mitochondrial membrane, but can be synthesized from citrate ( citrate shuttle ). Acetyl-CoA for the citric acid cycle can come from various sources, for example from the β-oxidation of fatty acids.
The subsequent isomerization 2a-b of the citrate by aconitase yields isocitrate . The importance of this step lies in the conversion of a difficult-to-oxidize tertiary alcohol (citrate) into an easy-to-oxidize secondary alcohol (isocitrate).
Isocitrate is oxidized and decarboxylated by the isocitrate dehydrogenase in steps 3a-b . In addition to the first reduction equivalent NADH, α-ketoglutarate (other name: 2-oxoglutarate) is formed, an intermediate product that is also important for amino acid metabolism ( cataplerotic metabolic pathway: reductive transamination to L - glutamate ⇒ amino acid biosynthesis; anaplerotic metabolic pathway: deamination of glutamate ⇒).
The following reaction 4 , which via oxidative decarboxylation yields a second molecule of CO 2 in addition to NADH , is catalyzed by the α-ketoglutarate dehydrogenase complex , which is functionally and structurally similar to the pyruvate dehydrogenase complex. Experiments with isotope-labeled substrates show that the CO 2 released in the process cannot be assigned to the carbon of the carbonyl group of the acetyl-CoAs, but, like that from step 3b, comes from the oxaloacetate.
The resulting succinyl-CoA is another key product of the citric acid cycle (cataplerotic metabolic pathway: porphyrin biosynthesis; anaplerotic metabolic pathways: degradation of the amino acids L - valine , L - isoleucine and L - methionine , oxidation of odd-numbered fatty acids , see also fatty acid oxidation ).
The means of the succinyl-CoA synthetase catalyzed hydrolysis of 5 of the high-energy thioester succinyl-CoA to succinate is the only supplies energy equivalent of the citric acid cycle in the form of GTP. A nucleoside diphosphate kinase can convert GTP into ATP.
In step 6, succinate is the substrate of succinate dehydrogenase , which provides a third reduction equivalent in the form of FADH 2 through oxidation , as well as fumarate , which also occurs through an anaplerotic metabolic pathway through the breakdown of the amino acids L - aspartic acid , L - phenylalanine and L - tyrosine in the citric acid cycle is fed in.
The fumarase catalyzes the stereospecific addition of water to the double bond of the fumarate in step 7 , so that L - malate is formed. Like isocitrate, malate, as a secondary alcohol, is difficult to oxidize. As a result, in step 8, the substrate of the first step, oxaloacetate, is resynthesized by the malate dehydrogenase with the recovery of NADH. This closes the cycle. Further metabolic pathways are linked to the oxaloacetate (cataplerotic: reductive transamination to aspartate ⇒ amino acid biosynthesis; anaplerotic: deamination of aspartate ⇒ amino acid oxidation).
| Substrate | Reactants / coenzymes |
enzyme | Reaction type | Inhibitors | Activators | Products / Coenzymes |
|
|---|---|---|---|---|---|---|---|
| 1 | Oxaloacetate | Acetyl-CoA, water | Citrate synthase | condensation | Citrate, NADH, succinyl-CoA, ATP | Citrate | |
| 2a | Citrate | - | Aconitase | Dehydration | cis aconitate, water | ||
| 2 B | cis aconitate | water | Hydration | Isocitrate | |||
| 3a | Isocitrate | NAD + | Isocitrate dehydrogenase | oxidation | NADH, ATP | Ca 2+ , ADP | Oxal succinate, NADH |
| 3b | Oxal succinate | H + | Decarboxylation | α-ketoglutarate, CO 2 | |||
| 4th | α-ketoglutarate | NAD + , CoA-SH | α-ketoglutarate dehydrogenase complex | Oxidative decarboxylation | NADH, succinyl-CoA | Ca 2+ | Succinyl-CoA, NADH, CO 2 |
| 5 | Succinyl-CoA | GDP, phosphate | Succinyl-CoA synthetase | Phosphate transfer | Succinate, GTP, CoA-SH | ||
| 6th | Succinate | FAD | Succinate dehydrogenase | oxidation | Malonate | Mg 2+ | Fumarate, FADH 2 |
| 7th | Fumarate | water | Fumarase | Hydration | L-malate | ||
| 8th | L -Malat | NAD + | Malate dehydrogenase | oxidation | Oxaloacetate, NADH | ||
| Not part of the citric acid cycle: | |||||||
| A. | Pyruvate | NAD + , CoA-SH | Pyruvate dehydrogenase complex | Oxidative decarboxylation | NADH, acetyl-CoA | Ca 2+ | Acetyl-CoA |
| B. | Pyruvate | ATP, H + , CO 2 | Pyruvate carboxylase | Carboxylation | Acetyl-CoA | Oxaloacetate, ADP, phosphate | |
regulation
The citric acid cycle as the central pivot point of aerobic metabolism is subject to strong regulatory influences. In addition to product inhibition (“negative feedback”, competitive inhibition ) and inhibition by other intermediate compounds, particularly NAD + / NADH, ADP / ATP and Ca 2+ play a major role as effectors . In particular, the sub-steps of major exergonia are subject to regulatory control : citrate synthesis 1 (ΔG o = −38 kJ / mol), ketoglutarate formation 3 (ΔG o = −7 kJ / mol) and the formation of succinyl-CoA 4 ( ΔG o = −37 kJ / mol).
The above-mentioned exergonic partial steps are inhibited by high NADH levels: B. The respiratory chain comes to a standstill due to a lack of oxygen, i.e. if less NADH is consumed and its concentration increases, the citric acid cycle can also come to a standstill.
On the other hand, if little energy is required (e.g. muscles at rest), the ATP concentration increases as the ADP concentration decreases. While ADP is an allosteric activator of isocitrate dehydrogenase, ATP inhibits its effect: the cycle is slowed down.
Further effectors of the citric acid cycle can be found in the table.
Inhibitors
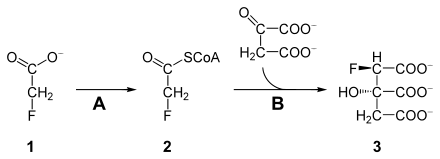
Fluoroacetate is toxic as it can block the citric acid cycle. Fluoroacetate ( 1 ) is first metabolized by an acetyl-CoA synthetase ( A , EC 6.2.1.1 ) to fluoroacetyl-CoA ( 2 ). Fluoroacetyl-CoA, like its analogue acetyl-CoA, is catalyzed by the citrate synthase ( B ) and condensed with oxaloacetate. The product, (2 R , 3 S ) -fluorocitrate ( 3 ), cannot be processed by the aconitase and blocks it, whereby the citric acid cycle breaks off at this point. The cell is cut off from the energy supply and dies (lethal synthesis).
Citric acid cycle in humans
In humans, too, sugars are broken down into CO 2 and H 2 O via glycolysis , the oxidative decarboxylation of pyruvate and the citric acid cycle with the formation of the energy carriers NADH + H + , FADH 2 , GTP and ATP . The energy of the energy carriers formed (except ATP) is transferred via the respiratory chain to ADP , which can then be built up into further ATP with the help of a phosphate residue . Here, NADH + H + releases approximately the energy that can be used to form 3 ATP, FADH 2 releases approximately the energy that is required to form 2 ATP, GTP provides energy to build up an ATP molecule ADP and phosphate.
With increased performance demand, due to a lack of oxygen, without which the respiratory chain cannot run, a growing percentage of the pyruvate obtained in the glycolysis is no longer aerobically converted to acetyl-CoA, but anaerobically with consumption of one NADH + H + per pyruvate molecule to L - Lactate , the anion of lactic acid. It seems incomprehensible that NADH + H + is consumed, since the body actually needs energy in this situation. On closer inspection, however, this step is necessary and energizing, because NADH + H + cannot be converted into ATP by the respiratory chain anyway (lack of oxygen). However, 2 ATP, which can be used directly by the muscles without the respiratory chain, can be formed in glycolysis by breaking down 1 molecule of glucose into 2 molecules of pyruvate. This also creates 2 molecules of NADH + H + , so that in the end there is an energy gain of 2 ATP. However, so that pyruvate formation can take place continuously, it must be ensured that pyruvate is removed from the system again and again (so that the concentration does not become too high), which would normally happen via decarboxylation and the citric acid cycle. Since this is not possible due to the lack of oxygen, as mentioned, pyruvate is broken down into lactate. So glycolysis can continue and at least 2 ATP are formed:
| Metabolic process | Energy balance |
|---|---|
| Conversion of 2 pyruvate to 2 lactate | −6 ATP (2 NADH + H + ) |
| Breakdown of 1 glucose to 2 pyruvate | +8 ATP (2 NADH + H + and 2 ATP) |
| Balance per glucose molecule | +2 ATP |
Lactic acid has to be broken down above a certain concentration because it has a performance-reducing effect by lowering the pH value . The muscles release lactate to the blood, which is transported to the liver. Lactate is then converted into glucose in the liver through the process of gluconeogenesis . More energy is required here than was absorbed by the muscle. The process of converting pyruvate to lactate is therefore only beneficial in the short term in terms of energy when viewed regionally on the muscle. For the organism as a whole, however, it means long-term energy losses (see also Cori cycle ). This shows that in extreme situations - here high performance requirements - the body can be prepared to forfeit energy in the long term in order to achieve the required performance in the short term.
The glucose formed in the liver can then be taken up again by the muscle cells through the blood. This cycle is also known as the Cori cycle . The ability to maintain high performance despite high lactate levels is referred to in physiologically based training theory as lactate tolerance .
→ see also: glycolysis , lactic acid fermentation
variants
Modified citric acid cycle metabolic pathways, in which a partial step is missing, are the norm in bacteria (13 of 17 examined). The missing step may or may not be replaced by other reaction steps. In fact, only two types of bacteria are known to have ketoglutarate dehydrogenase (KDH) activity: Bacillus japonicum and Escherichia coli . The bacterium Escherichia coli runs the complete citric acid cycle as described under aerobic conditions. Under anaerobic conditions it is able to deactivate the KDH. The metabolic pathways that previously formed a circle are now linked like a tree. M. tuberculosis, on the other hand, can switch between two different citric acid cycles, both of which are different from the eukaryotic pathway.
Archaea , but also some bacteria, such as Helicobacter pylori , which grows under microaerophilic conditions, catalyze the conversion of α-ketoglutarate to succinyl-CoA using an oxidation-sensitive 2-ketoglutarate: ferredoxin oxidoreductase (OGOR, EC 1.2.7.3 ). In contrast to the OGDC, this one contains iron-sulfur clusters ; the flavin and lipoic acid amide are missing. Instead of NADH, ferredoxin is used as a reduction equivalent. Also Mycobacterium tuberculosis contains a CoA-dependent enzyme that, however, is stable even under aerobic conditions.
In various mycobacteria (including Mycobacterium tuberculosis ), the E1 subunit of ketoglutarate dehydrogenase has been replaced by a ketoglutarate decarboxylase, which, independently of coenzyme A, initially produces succinate semialdehyde, which is converted from an NADP + -dependent succinate semialdehyde to succinate dehydrogenase becoming dehydrated.
reversal
In some bacteria, the citric acid cycle is operated in the reverse order for carbon dioxide assimilation ( reductive citric acid cycle ). Here, with the consumption of ATP and the use of reducing agents, three energetically unfavorable steps of the oxidative citric acid cycle are bypassed: the citrate synthase is replaced by an ATP citrate lyase, the α-ketoglutarate dehydrogenase by an α-ketoglutarate synthase and the succinate -Dehydrogenase by a fumarate reductase.
In 2018, research groups in two thermophilic, sulfur-reducing, anaerobic bacteria ( Desulfurella acetivorans and Thermosulfidibacter takaii ) discovered that they use a reverse order of the citric acid cycle, but do not encode a gene for the ATP-dependent citrate lyase necessary in the reductive citric acid cycle. This metabolic pathway was called the reverse or reverse oxidative citric acid cycle (“roTCA”) to distinguish the reductive citric acid cycle (“rTCA”). The bacteria succeed in reversing the formation of citrate by efficiently metabolizing the successor metabolites malate and acetyl-CoA; as a result, the concentration of oxaloacetate is extremely low, so that the equilibrium of citrate cleavage is shifted to the side of oxaloacetate. By saving one molecule of ATP, only one molecule of ATP is required in the roTCA to fix two molecules of CO 2 . This corresponds to the energy requirements of the reductive acetyl-CoA pathway .
See also
literature
- Reginald Garrett, Charles M. Grisham: Biochemistry . International Student Edition. 4th edition. Cengage Learning Service, Australia 2009, ISBN 0-495-11464-2 , pp. 563ff.
- Geoffrey Zubay: biochemistry . 4th ed. Mcgraw-Hill International, London 1999, ISBN 3-89028-701-8 .
- Donald Voet, Judith G. Voet: Biochemistry . Wiley-VCH, Weinheim 1994, ISBN 3-527-29249-7 .
- Jeremy M. Berg, John L. Tymoczko, Lubert Stryer: Biochemistry . 6th ed. Spectrum, Heidelberg 2007, ISBN 3-8274-1800-3 .
- H. Robert Horton, Laurence A. Moran, K. Gray Scrimgeour, Marc D. Perry, J. David Rawn, Carsten Biele (trans.): Biochemistry . 4th edition. Pearson Studium, Munich 2008. ISBN 3-8273-7312-3 .
- David L. Nelson, Michael M. Cox, Albert L. Lehninger (first): Lehninger Biochemie . 4th edition Springer, Berlin 2009, ISBN 3-540-68637-1 .
Web links
- What is the goal of the citrate cycle? ( Memento from May 2, 2016 in the Internet Archive )
Individual evidence
- ^ Nobel Prize in Medicine 1953 .
- ^ Cancer, HA. and Johnson, WA. (1937): The role of citric acid in intermediate metabolism in animal tissues . In: Enzymologia 4, 148-156 (reprint: HA Krebs, WA Johnson: The role of citric acid in intermediate metabolism in animal tissues . In: FEBS letters . 117 Suppl, 25 August 1980, p. K1-10 , PMID 6998725 . )
- ^ Albert L. Lehninger, David L. Nelson, Michael M. Cox: Lehninger Biochemie . 3rd edition Springer, Berlin 2009, ISBN 3-540-41813-X , p. 626.
- ↑ Hans Günther Schlegel, Georg Fuchs (Ed.): General microbiology . 8th edition Thieme, Stuttgart 2006, ISBN 3-13-444608-1 , p. 326.
- ^ Reginald Garrett, Charles M. Grisham: Biochemistry. International Student Edition. 4th edition. Cengage Learning Services, Australia 2009, ISBN 0-495-11464-2 , p. 573.
- ↑ Marc W. van der Kamp, John D. McGeagh, Adrian J. Mulholland: “Lethal Synthesis” of Fluorocitrate by Citrate Synthase Explained through QM / MM Modeling . In: Angewandte Chemie International Edition . tape 50 , no. 44 , October 24, 2011, ISSN 1521-3773 , p. 10349-10351 , doi : 10.1002 / anie.201103260 .
- ↑ SJ Cordwell: Microbial genomes and “missing” enzymes: redefining biochemical pathways . In: Archives of Microbiology . tape 172 , no. 5 , October 1, 1999, p. 269-279 , doi : 10.1007 / s002030050780 , PMID 10550468 .
- ↑ May X, MW Adams: Characterization of a fourth type of 2-keto acid-oxidizing enzyme from a hyperthermophilic archaeon: 2-ketoglutarate ferredoxin oxidoreductase from Thermococcus litoralis . In: Journal of Bacteriology . tape 178 , no. October 20 , 1996, p. 5890-5896 , PMID 8830683 , PMC 178443 (free full text).
- ↑ SM Pitson, GL Mendz, S. Srinivasan, SL Hazell: The tricarboxylic acid cycle of Helicobacter pylori . In: European Journal of Biochemistry / FEBS . tape 260 , no. 1 , February 1999, p. 258-267 , PMID 10091606 .
- ↑ Anthony D. Baughn, Scott J. Garforth, Catherine Vilchèze, William R. Jacobs: An anaerobic-type alpha-ketoglutarate ferredoxin oxidoreductase completes the oxidative tricarboxylic acid cycle of Mycobacterium tuberculosis . In: PLoS pathogens . tape 5 , no. November 11 , 2009, p. e1000662 , doi : 10.1371 / journal.ppat.1000662 , PMID 19936047 .
- ↑ Jing Tian, Ruslana Bryk, Manabu Itoh, Makoto Suematsu, Carl Nathan: Variant tricarboxylic acid cycle in Mycobacterium tuberculosis: identification of alpha-ketoglutarate decarboxylase . In: Proceedings of the National Academy of Sciences . tape 102 , no. 30 , July 26, 2005, p. 10670-10675 , doi : 10.1073 / pnas.0501605102 , PMID 16027371 .
- ↑ Achim Mall et al .: Reversibility of citrate synthase allows autotrophic growth of a thermophilic bacterium . In: Science (New York, NY) . tape 359 , no. 6375 , February 2, 2018, p. 563-567 , doi : 10.1126 / science.aao2410 , PMID 29420287 .
- ↑ Takuro Nunoura et al .: A primordial and reversible TCA cycle in a facultatively chemolithoautotrophic thermophile . In: Science (New York, NY) . tape 359 , no. 6375 , February 2, 2018, p. 559-563 , doi : 10.1126 / science.aao3407 , PMID 29420286 .
- ↑ Stephen W. Ragsdale: Stealth reactions driving carbon fixation . In: Science (New York, NY) . tape 359 , no. 6375 , February 2, 2018, p. 517-518 , doi : 10.1126 / science.aar6329 , PMID 29420277 .