Zinc transporter ZIP9
| SLC39A9 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | SLC39A9, ZIP-9, ZIP9, solute carrier family 39 member 9 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | MGI: 1914820 HomoloGene: 6935 GeneCards: SLC39A9 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Zinc transporter ZIP9 also known as Zrt- and Irt-like protein 9 (ZIP9) and solute carrier family 39 member 9 (SLC39A9) is a protein that in humans is encoded by the SLC39A9 gene.[5] This protein is the 9th member out of 14 ZIP family proteins, which is a membrane androgen receptor (mAR) coupled to G proteins, and also classified as a zinc transporter protein.[5][6][7][8] ZIP family proteins are known by their function which is transporting zinc metal from the extracellular environment into the cells through cell membrane and via selective transport.[6]
Classification and nomenclature
Mammalian cells have two major groups of zinc transporter proteins; the ones that export zinc from the cytoplasm to the extracellular space (efflux), which are called ZnT (SLC30 family) , and ZIP (SLC39 family) proteins whose functions are in the opposite direction (influx).[9] ZIP family proteins are named as Zrt- and Irt-like proteins because of their similarities to Zrt and Irt proteins which are respectively zinc and iron -regulated transporter proteins in yeast and Arabidopsis that were discovered earlier than ZIP and ZnT proteins.[9] ZIP family is consisted of four subfamilies (I, II, LIV-1, and gufA), and ZIP9 is the only member of subfamily I.[10]
Isoforms
ZIP9 can be present as 3 different isoforms in human cells. The canonical isoform of this protein has a length of 307 amino acids, with a molecular mass of 32,251Da. In the second isoform, amino acids 135-157 are missing, so its length and molecular weight are respectively reduced to 284 amino acids and 29,931Da. In the third isoform the amino acids 233-307 are missing, so the isoform only has 232 amino acids and its molecular mass is 24,626 Da. Additionally, the last amino acid of isoform 3, which is usually serine, is replaced with aspartic acid.[11]
| Isoform | number of amino acids | size (Da) | transformation | missing amino acids |
|---|---|---|---|---|
| isoform 1 | 307 | 32251 | N/A | N/A |
| isoform 2 | 284 | 29931 | N/A | 135-157 |
| isoform 3 | 232 | 24626 | S -----> D | 233-307 |
Discovery
ZIP9 membrane androgen receptor was first discovered in Atlantic croaker (Micropogonias undulatus) brain, ovary and testicular tissues and named "AR2" in 1999, together with another androgen receptor which was found only in brain tissue, and it was named "AR1" in that time.[12] AR1 and AR2 were first thought to be nuclear androgen receptors (nAR), however, further studies on their biochemical and functional features in 2003 illustrated that they were involved in non-genomic mechanisms in the plasma membrane of the cells and were membrane androgen receptors.[13] In 2005, the similarities between the nucleotide and amino acid sequences of AR2 and ZIP family proteins were discovered in other vertebrates, suggesting that AR2 is from this family of proteins.[14] A study in 2014 utilised the latest research technologies to clone and express a particular cDNA of the female Atlantic croaker ovaries, which encoded a protein showing the characteristics of the canonical isoform of ZIP9, as a novel membrane androgen receptor(mAR).[7]
Structure
Unlike other ZIP subfamilies that are consisted of 8 transmembrane (TM) domains with an extracellular C-terminal, ZIP9 is consisted of a 7 TM structure with an intracellular C-terminus.[7] ZIP9 is shorter than other ZIP proteins, and only has about 307 amino acids within its structure, however, like other ZIP proteins, between its domains III and IV, within the intracellular loop, it contains histidine-rich clusters.[7] ZIP9 and other ZIP proteins have polar or charged amino acids in their TM domains which probably play important roles in making ion transfer channels and therefore in importing zinc ions into cytoplasm.[14]
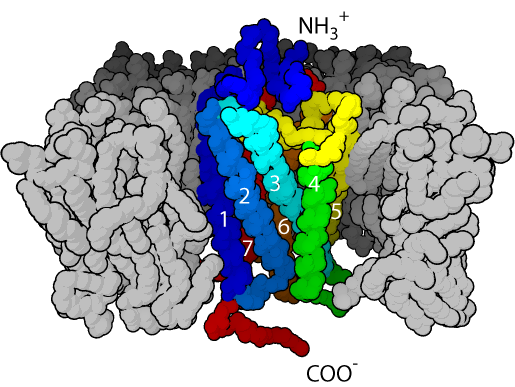
Location, expression and function
ZIP9 influxes zinc ions into the cytosol and its gene is expressed almost in every tissue of human body. [8] The sub-cellular location of ZIP9 is in plasma, nucleus, endoplasmic reticulum and mitochondrial membrane.[8] One of the responsibilities of ZIP9 is the homeostasis of zinc in the secretory pathway, during which this protein stays within the Trans Golgi Network regardless of the change in the concentrations of zinc.[10]

ZIP9 is the only ZIP protein that signals through G protein binding, and pharmaceutical agents decrease their ligand binding once ZIP9 is uncoupled from G proteins.[5] ZIP9 is also the only member of ZIP family with mAR characteristics.[5] In contrast to testosterone, which has high affinity for ZIP9 with a Kd of 14 nM, the other endogenous androgens dihydrotestosterone (DHT) and androstenedione show low affinity for the receptor with less than 1% of that of testosterone, although DHT is still effective in activating the receptor at sufficiently high concentrations.[5] Moreover, the synthetic androgens mibolerone and metribolone (R-1881), the endogenous androgen 11-ketotestoterone, and the other steroid hormones estradiol and cortisol are all ineffective competitors for the receptor.[5] As such, mibolerone and metribolone could potentially be employed to differentiate between androgen receptor- and ZIP9-mediated responses of testosterone.[5]
Clinical significance
Zinc homeostasis is very important in human health, because zinc is present in the structure of some proteins like zinc-dependent metalloenzymes and zinc-finger-containing transcriptional factors.[16] In addition, zinc is involved in signalling for cell growth, proliferation, division and apoptosis.[16][17] As a result, any dysfunction of zinc transporter proteins can be harmful for the cells, and some of them are associated with different cancers, diabetes and inflammation.[16] For instance, through activation of ZIP9, testosterone has been found to increase intracellular zinc levels in breast cancer, prostate cancer, and ovarian follicle cells and to induce apoptosis in these cells, an action which may be mediated partially or fully by increased zinc concentrations.[5][18]
Cancer
Breast and prostate
A study in 2014, elucidated the intermediary role of ZIP9 in causing human breast and prostate cancer, as it inducted the apoptosis of testosterone in breast and prostate cancerous cells.[8] unlike ZIP1, 2 and 3, ZIP9 mRNA expression was increased in human prostate and breast malignant biopsy cancer cells, which probably was because cells that divide rapidly require more zinc. [8]
Brain
Treatment of glioblastoma cells with TPEN showed that upregulation of ZIP9 in glioblastoma cells enhances cell migration in brain cancer by influencing P53 and GSK-3ß, and also ERK and AKT signalling pathways in phosphorylation after activation of B-cell receptors.[19]
Diabetes
Zinc must be constantly supplied to Pancreatic β-cells to function normally and maintain glycaemic control.[17] The insulin secretory pathway in humans is highly dependent on zinc activities.[20] The cells lose many zinc ions during the secretion of insulin, and need to receive more zinc, and interestingly expression of ZIP9 mRNA during this process increases.[21] As a result, ZIP9, which is involved in importing zinc into the cells, is potentially a target for therapeutic studies in the future regarding diabetes type2. [21]
See also
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000029364 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000048833 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ a b c d e f g h Thomas P, Converse A, Berg HA (May 2017). "ZIP9, a novel membrane androgen receptor and zinc transporter protein". General and Comparative Endocrinology. doi:10.1016/j.ygcen.2017.04.016. PMID 28479083.
- ^ a b Eide DJ (February 2004). "The SLC39 family of metal ion transporters". Pflugers Archiv. 447 (5): 796–800. doi:10.1007/s00424-003-1074-3. PMID 12748861.
- ^ a b c d Berg AH, Rice CD, Rahman MS, Dong J, Thomas P (November 2014). "Identification and characterization of membrane androgen receptors in the ZIP9 zinc transporter subfamily: I. Discovery in female atlantic croaker and evidence ZIP9 mediates testosterone-induced apoptosis of ovarian follicle cells". Endocrinology. 155 (11): 4237–49. doi:10.1210/en.2014-1198. PMC 4197986. PMID 25014354.
- ^ a b c d e Thomas P, Pang Y, Dong J, Berg AH (November 2014). "Identification and characterization of membrane androgen receptors in the ZIP9 zinc transporter subfamily: II. Role of human ZIP9 in testosterone-induced prostate and breast cancer cell apoptosis". Endocrinology. 155 (11): 4250–65. doi:10.1210/en.2014-1201. PMC 4197988. PMID 25014355.
- ^ a b Lichten LA, Cousins RJ (2009-07-22). "Mammalian zinc transporters: nutritional and physiologic regulation". Annual Review of Nutrition. 29 (1): 153–76. doi:10.1146/annurev-nutr-033009-083312. PMID 19400752.
- ^ a b Matsuura W, Yamazaki T, Yamaguchi-Iwai Y, Masuda S, Nagao M, Andrews GK, Kambe T (May 2009). "SLC39A9 (ZIP9) regulates zinc homeostasis in the secretory pathway: characterization of the ZIP subfamily I protein in vertebrate cells". Bioscience, Biotechnology, and Biochemistry. 73 (5): 1142–8. doi:10.1271/bbb.80910. PMID 19420709.
- ^ a b Universal protein resource accession number Q9NUM3 at UniProt.
- ^ Sperry TS, Thomas P (April 1999). "Characterization of two nuclear androgen receptors in Atlantic croaker: comparison of their biochemical properties and binding specificities". Endocrinology. 140 (4): 1602–11. doi:10.1210/endo.140.4.6631. PMID 10098494.
- ^ Braun AM, Thomas P (November 2003). "Androgens inhibit estradiol-17beta synthesis in Atlantic croaker (Micropogonias undulatus) ovaries by a nongenomic mechanism initiated at the cell surface". Biology of Reproduction. 69 (5): 1642–50. doi:10.1095/biolreprod.103.015479. PMID 12855603.
- ^ a b Eide DJ (2005). "The Zip Family of Zinc Transporters". In Iuchi S, Kuldell N (eds.). Zinc Finger Proteins. Boston, MA: Molecular Biology Intelligence Unit. Springer. doi:10.1007/0-387-27421-9_35.
- ^ Zhao L, Xia Z, Wang F (2014). "Zebrafish in the sea of mineral (iron, zinc, and copper) metabolism". Frontiers in Pharmacology. 5: 33. doi:10.3389/fphar.2014.00033. PMC 3944790. PMID 24639652.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c Taniguchi M, Fukunaka A, Hagihara M, Watanabe K, Kamino S, Kambe T, Enomoto S, Hiromura M (2013). "Essential role of the zinc transporter ZIP9/SLC39A9 in regulating the activations of Akt and Erk in B-cell receptor signaling pathway in DT40 cells". PloS One. 8 (3): e58022. doi:10.1371/journal.pone.0058022. PMC 3591455. PMID 23505453.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Li YV (March 2014). "Zinc and insulin in pancreatic beta-cells". Endocrine. 45 (2): 178–89. doi:10.1007/s12020-013-0032-x. PMID 23979673.
- ^ Pascal LE, Wang Z (November 2014). "Unzipping androgen action through ZIP9: a novel membrane androgen receptor". Endocrinology. 155 (11): 4120–3. doi:10.1210/en.2014-1749. PMID 25325426.
- ^ Münnich N, Wernhart S, Hogstrand C, Schlomann U, Nimsky C, Bartsch JW (December 2016). "Expression of the zinc importer protein ZIP9/SLC39A9 in glioblastoma cells affects phosphorylation states of p53 and GSK-3β and causes increased cell migration". Biometals. 29 (6): 995–1004. doi:10.1007/s10534-016-9971-z. PMID 27654922.
- ^ Huang L. "Zinc and its transporters, pancreatic β-cells, and insulin metabolism". Vitamins and Hormones. 95: 365–90. doi:10.1016/b978-0-12-800174-5.00014-4. PMID 24559925.
- ^ a b Lawson R, Maret W, Hogstrand C (September 2017). "Expression of the ZIP/SLC39A transporters in β-cells: a systematic review and integration of multiple datasets". BMC Genomics. 18 (1): 719. doi:10.1186/s12864-017-4119-2. PMID 28893192.
{{cite journal}}: CS1 maint: unflagged free DOI (link)




