2-azetidinone
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 2-azetidinone | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 3 H 5 NO | |||||||||||||||
| Brief description |
colorless to beige solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 71.08 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density | ||||||||||||||||
| Melting point | ||||||||||||||||
| boiling point | ||||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
2-Azetidinone is the cyclic amide of the non-proteinogenic amino acid β-alanine and the simplest, four-membered β- lactam . 2-Azacyclobutanones are lead structures in β-lactam antibiotics , such as the monocyclic monobactams and especially in the fused bicyclic penam and cephem skeletons on which the penicillins and cephalosporins are based. As a lactam monomer, 2-azacyclobutanone can be anionically polymerized to poly (β-alanine) or polyamide 3.
Occurrence and representation
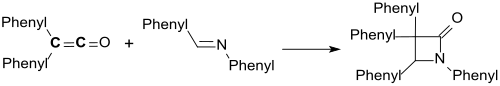
The synthesis of (phenyl-substituted) 2-azetidinone by (2 + 2) cycloaddition of diphenylketene to benzalaniline was first described in 1907 by Hermann Staudinger .
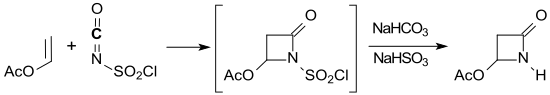
The compound 4-acetoxy-2-azetidinone , which can be obtained from vinyl acetate and chlorosulfonyl isocyanate in a (2 + 2) cycloaddition in yields of 44–62%, opens up good access to the target molecule 2-azacyclobutanone .
In the reduction of 4-acetoxy-2-azetidinone with potassium borohydride in water, 2-azetidinone is obtained in 20% yield after short-path distillation .
The analogous cycloaddition of ethylene with chlorosulfonyl isocyanate with subsequent hydrolysis for the synthesis of the unsubstituted 2-azetidinone does not lead to the desired end product 2-azacyclobutanone because of the inertia of ethylene.
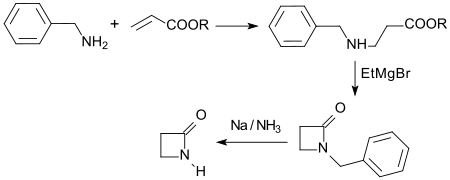
The unsubstituted 2-azetidinone was obtained for the first time in 1949 by cyclization of β-alanine ethyl ester with ethyl magnesium bromide in diethyl ether after elaborate isolation and purification in a total yield of only 0.76%.
The Michael addition of benzylamine to acrylic acid ester to form N-benzyl-β-alanine ester, which can be cyclized with ethylmagnesium bromide to form N-benzyl-β-propiolactam, is much more efficient. The reductive cleavage of the benzyl radical with sodium in liquid ammonia gives 2-azetidinone in an overall yield of 50%.
properties
2-Azetidinone is a colorless and odorless solid that dissolves easily in polar solvents such as water and ethanol. Dilute sodium hydroxide hydrolyzes the lactam to the β-alanine sodium salt. β-propiolactam is much less reactive than propiolactone , which reacts completely with methanol at 48 ° C without a catalyst to form β-methoxypropionic acid, while 2-azetidinone shows no reaction.
Applications
Building block in pharmacologically active ingredients
The four-membered lactam ring system of 2-azetidinone is a common structural feature of a number of β-lactam antibiotics , such as. B. the penicillins, cephalosporins, and carbapenems , the β-lactamase inhibitors sulbactam , tazobactam and clavulanic acid and the non-fused monobactams aztreonam , tigemonam and carumonam . Azetidin-2-one itself has no antibiotic activity.
The 2-azetidinone skeleton is also found in the cholesterol uptake inhibitor ezetimibe and in numerous experimental active substances, e.g. B. trypsin - or thrombin - enzyme inhibitors , antidiabetics , analgesics , vasopressin antagonists or Parkinson agents .
Monomer for poly (β-alanine)
The anionic ring-opening polymerization (ROP) of 2-azetidinone yields high molecular weight poly (β-alanine), which can not be obtained by polycondensation of β-alanine.
In contrast to polyamide 6 , which is accessible from seven-membered ε- caprolactam through ROP , poly (β-alanine), also known as polyamide 3 or nylon 3, only melts at temperatures above 340 ° C with considerable decomposition and can therefore not be processed thermoplastically. From solution in formic acid , poly (β-alanine) can be processed into films and fibers that have not yet been used industrially. The immobilized lipase from Candida antarctica (CALB) catalyzes the ROP of 2-azetidinone in toluene , although only average degrees of polymerization DP of 8 are achieved. 2-Azetidinone is of little practical interest because of its comparatively complex production and the limited usefulness of the polymeric nylon 3.
Individual evidence
- ↑ a b c d e f g R.W. Holley, AD Holley: 2-Azetidinone (β-Propiolactam) . In: J. Am. Chem. Soc. tape 71 , no. 6 , 1949, pp. 2129-2131 , doi : 10.1021 / ja01174a062 .
- ↑ a b c d e data sheet 2-Azetidinone from Sigma-Aldrich , accessed on February 5, 2019 ( PDF ).
- ^ Carl L. Yaws: Thermophysical Properties of Chemicals and Hydrocarbons, 2nd Edition . Elsevier Inc., Amsterdam, NL 2014, ISBN 978-0-323-28659-6 , pp. 268 .
- ^ William M. Haynes: CRC Handbook of Chemistry and Physics, 97th Edition . CRC Press, Boca Raton, FL, USA 2016, ISBN 978-1-4987-5429-3 , pp. 3-32 .
- ↑ U. Holzgrebe: Structure-activity relationships, From penicillin G to tricyclic β-lactams . In: Pharmacy in our time . tape 35 , no. 5 , 2006, p. 410-414 , doi : 10.1002 / 200600186 .
- ↑ a b c d e H. Bestian: About poly-β-amides . In: Angew. Chem. Band 80 , no. 8 , 1968, p. 304-312 , doi : 10.1002 / anie.19680800803 .
- ↑ H. Staudinger: On the knowledge of the ketenes. Diphenyl ketenes . In: Liebigs Ann. Chem. Band 356 , no. 1-2 , 1907, pp. 51-123 , doi : 10.1002 / jlac.19073560106 .
- ↑ K. Clauß, D. Grimm, G. Prossel: β ‐ lactams with substituents bound via heteroatoms . In: Liebigs Ann. Chem. Band 1974 , no. 4 , 1974, p. 539-560 , doi : 10.1002 / jlac.197419740403 .
- ^ SJ Mickel, S.-N Hsiao, MJ Miller: Synthesis of a key β-lactam intermediate by a [2 + 2] cycloaddition route: 4-Acetoxyazetidin-2-one In: Organic Syntheses . 65, 1987, p. 135, doi : 10.15227 / orgsyn.065.0135 ; Coll. Vol. 8, 1993, p. 3 ( PDF ).
- ↑ a b c L.W. Schwab, R. Kroon, AJ Schouten, K. Loos: Enzyme-catalyzed ring-opening polymerization of unsubstituted β ‐ lactam . In: Macromol. Rapid Commun. tape 29 , no. 10 , 2008, p. 794-797 , doi : 10.1002 / marc.200800117 .
- ↑ PD Mehta, NPS Sengar, AK Pathak: 2-Azetidinone - A new profile of various pharmacological activities . In: Eur. J. Med. Chem. Volume 45 , 2010, p. 5541-5560 , doi : 10.1016 / ejmech.2010.09.035 .