Acinetobacter baumannii
| Acinetobacter baumannii | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
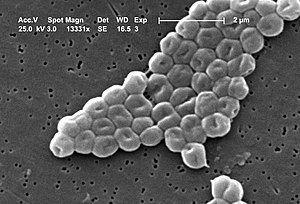
electron micrograph of Acinetobacter baumannii |
||||||||||||
| Systematics | ||||||||||||
|
||||||||||||
| Scientific name | ||||||||||||
| Acinetobacter baumannii | ||||||||||||
| Bouvet & Grimont 1986 |
Acinetobacter baumannii is a gram-negative , aerobic bacterium. The genome of the Acinetobacter baumannii AF-401strainwas completely sequenced in 2017. Like other species of the genus Acinetobacter , the often multi-resistant (gram-negative) bacterium is one of the causes of nosocomial infections . In many countries, A. baumannii is one of the most important hospital germs and occurs mainly in intensive care units. The American Society for Infectious Diseases counts Acinetobacter baumannii asone of the so-called ESKAPE pathogens, against which therapeutic options are scarce,due to its abilityto develop particularly pronounced antibiotic resistance .
features
Appearance
The cells of Acinetobacter baumannii are not actively motile by flagella . They are rod-shaped , but cocci-shaped cells also occur in the stationary growth phase . A. baumannii is one of the Gram- negative bacteria, but the Gram stain is often ambiguous ( Gram- variable) and shows cocoid rods in the light microscope image. There are no persistence forms such as endospores . On casein soy peptone agar , the bacterial colonies grow circular, convex, smooth and slightly opaque. They have a diameter of 1.5 to 2.0 mm after 24 hours of incubation and 3.0 to 4.0 mm after 48 hours of incubation at 30 ° C.
Growth and metabolism
Acinetobacter species are negative in the oxidase test , which distinguishes them from Pseudomonas from the same order Pseudomonadales. They belong to the group of non-fermenting bacteria (so-called nonfermenters ).
The growth takes place between 15 and 44 ° C. Most strains can form acid from glucose . There is no hemolysis on blood agar , and gelatin cannot be hydrolyzed . On Simmons citrate agar , prototrophic strains can utilize citrate , auxotrophs cannot, unless they are additionally supplied with growth factors in the nutrient medium. Additional metabolic properties are listed in the Evidence section .
genetics
The genome of the Acinetobacter baumannii AF-401 strain was completely sequenced in 2017 . This is a project for genome sequencing of Carbapenem -resister A. baumannii strains. The genome of more than 20 strains has now been researched. The genome has a size of 3982 kilobase pairs (kb), 3953 proteins are annotated . The results of the sequencing show a GC content (the proportion of the nucleobases guanine and cytosine ) in the bacterial DNA of 39 mol percent ( median ). This is somewhat below the GC content of 40-43 mol percent stated in the first description. In addition to the bacterial chromosome, there is a plasmid on which several antibiotic resistance genes are located. The plasmid and thus also the antibiotic resistance can be exchanged between bacterial species by means of horizontal gene transfer .
proof
The criterion “Growth at 42 ° C” can be used to distinguish Acinetobacter calcoaceticus , which does not grow at this temperature.
Biochemical evidence
Biochemical features, such as the enzymes present and the resulting metabolic properties, can be used in a colored row to identify Acinetobacter baumannii or to differentiate between the Acinetobacter species or to distinguish them from other Gram-negative bacteria. There is no formation of hydrogen sulfide (H 2 S), the indole formation is also negative. The enzyme β-galactosidase to break down lactose is also absent. The substrates that A. baumannii can utilize include, for example, D - and L - lactate , glutarate , L - aspartate , L - tyrosine , 4-aminobutyrate , ethanol and 2,3-butanediol .
Miniaturized test systems can be used for biochemical identification ; systems for gram-negative rods ( Enterobacteriaceae ) or gram-negative rods (non-Enterobacteriaceae) are suitable . The distinction between the two species A. baumannii and A. calcoaceticus is possible with additional tests. Species diagnosis is difficult, however.
Further evidence
By MALDI-TOF MS is a reliable differentiation between different Acinetobacter spp. possible. The species Acinetobacter baumannii can also be reliably determined by partial gene sequencing using the rpoB locus or by means of PCR using the bla OXA-51-like gene .
Occurrence
A. baumannii is an environmental germ and occurs in soil and water, but numerous isolates also come from clinical samples from human patients. Acinetobacter strains with the carbapenemase NDM-1 have meanwhile also been isolated from clinical wastewater and poultry meat.
Systematics and taxonomy
The epithet was chosen in honor of Paul and Linda Baumann (American bacteriologists). The type strain of Acinetobacter baumannii was deposited in the collections of microorganisms in the USA (as ATCC 19606), Germany (at the DSMZ as DSM 30007) and other countries.
The two species A. baumannii and A. calcoaceticus are closely related, as shown by phenotypic test results and genetic studies with the help of DNA-DNA hybridization . In medical diagnostics, they are therefore referred to as the A. calcoaceticus A.baumannii complex (ACB complex). The species Acinetobacter nosocomialis (formerly genome species 13TU) and Acinetobacter pittii (formerly genome species 3) are also included.
Medical importance
In many countries, A. baumannii is one of the most important hospital germs and occurs mainly in intensive care units. The American Society for Infectious Diseases counts Acinetobacter baumannii as one of the so-called ESKAPE pathogens, against which therapeutic options are scarce, due to its ability to develop particularly pronounced antibiotic resistance . Acinetobacter baumannii is able to form biofilms and also to withstand drought. Surfaces around populated patients are therefore often contaminated . The transmission potential clearly exceeds that of classic hospital germs such as MRSA .
Clinical manifestations
The nosocomial infections caused by Acinetobacter baumannii manifest themselves in the form of pneumonia (also caused by ventilation), catheter-associated infections, bacteremia and sepsis. Wound infections (e.g. after burns) and nosocomial urinary tract infections and abscesses can also occur. Meningitis is also rare (e.g. after neurosurgery).
Pathogenicity
A. baumannii is assigned to risk group 2 by the Biological Agents Ordinance in conjunction with the TRBA ( Technical Rules for Biological Agents) 466 .
Antibiotic resistance
Acinetobacter baumannii is one of the multi-resistant gram-negative rod bacteria and is often classified as 3 MRGN or even 4MRGN ( KRINKO definition).
outbreaks
High profile acquired Acinetobacter baumannii by his frequent detection in infected wounds of US soldiers from missions in Iraq and Afghanistan returned home.
In July 2008, 23 cases were reported in the intensive care unit for severe burn injuries at the Hannover Medical School . The A. baumanii strain was only sensitive to one antibiotic. The resistant pathogen was eliminated from the station through consistently increased hygiene measures.
At the beginning of 2015 there was an outbreak at the University Hospital Schleswig-Holstein Campus Kiel, in which Acinetobacter baumannii was detected in 31 patients, 12 patients died and in 3 the infection could have been the direct cause of death. The cause for concern was the fact that it was a multi-resistant 4MRGN pathogen.
therapy
Acinetobacter baumannii infections are treated with imipenem or meropenem and (depending on the antibiogram with) quinolone or aminoglycoside . Alternatively (if sensitivity is proven) tigecycline and amikacin or ciprofloxacin can also be used. Resistant strains can be treated with colistin and meropenem or imipenem. In the case of carbapenem resistance, a combination of colistin and amikacin can be considered.
In 2019, a potentially effective antibiotic against Acinetobacter baumannii was found with the molecule halicin .
Food spoilage
Acinetobacter baumannii can lead to deviations in odor on fresh meat .
Individual evidence
- ^ A b Jean Euzéby, Aidan C. Parte: Genus Acinetobacter. In: List of Prokaryotic names with Standing in Nomenclature, Systematics of Bacteria (LPSN) . Retrieved April 4, 2018 .
- ↑ a b c d e f g h i j Acinetobacter baumannii - a hospital germ with unsettling development potential . In: Robert Koch Institute (Ed.): Epidemiologisches Bulletin 32/2013 . August 12, 2013, p. 295–299 ( Online + PDF, 135 KB [accessed April 4, 2018]).
- ↑ a b c Michael T. Madigan, John M. Martinko, Jack Parker: Brock Mikrobiologie. German translation edited by Werner Goebel, 1st edition. Spektrum Akademischer Verlag GmbH, Heidelberg / Berlin 2000, ISBN 3-8274-0566-1 , pp. 530-531.
- ↑ a b c d e f g h Philippe JM Bouvet, Patrick AD Grimont: Taxonomy of the Genus Acinetobacter with the Recognition of Acinetobacter baumannii sp. nov., Acinetobacter haemolyticus sp. nov., Acinetobacter johnsonii sp. nov., and Acinetobacter junii sp. nov. and Emended Descriptions of Acinetobacter calcoaceticus and Acinetobacter lwoffii . In: International Journal of Systematic and Evolutionary Microbiology . tape 36 , no. 2 , 1986, p. 228-240 , doi : 10.1099 / 00207713-36-2-228 .
- ↑ Acinetobacter baumannii AF-401. In: JCI Genomes Online Database (GOLD) website . Retrieved April 4, 2018 .
- ↑ Acinetobacter baumannii. In: National Center for Biotechnology Information (NCBI) Genome website . Retrieved April 4, 2018 .
- ↑ API® ID test strips. (Online + PDF, 2.1 MB) In: website of bioMérieux Deutschland GmbH. Retrieved April 4, 2018 .
- ↑ Marianne Abele-Horn (2009), p. 260.
- ↑ TRBA (Technical Rules for Biological Agents) 466: Classification of prokaryotes (Bacteria and Archaea) into risk groups. In: Website of the Federal Institute for Occupational Safety and Health (BAuA). August 25, 2015, accessed on March 29, 2018 (last update March 31, 2017).
- ↑ a b Christine Starostzik: Problem germ challenges hospital staff. In: Doctors newspaper. February 2, 2015, accessed April 4, 2018 .
- ^ Nicola Siegmund-Schultze: Acinetobacter baumanii: With this germ in the clinic, there is fire under the roof. In: Doctors newspaper. June 29, 2010, accessed April 4, 2018 .
- ^ Marianne Abele-Horn: Antimicrobial Therapy. Decision support for the treatment and prophylaxis of infectious diseases. With the collaboration of Werner Heinz, Hartwig Klinker, Johann Schurz and August Stich, 2nd, revised and expanded edition. Peter Wiehl, Marburg 2009, ISBN 978-3-927219-14-4 , p. 260.
- ↑ Julia Merlot, DER SPIEGEL: Fighting Resistance: Artificial Intelligence Discovers Promising Antibiotic - DER SPIEGEL - Science. Retrieved February 22, 2020 .
- ↑ Arthur Hinton Jr., JA Cason, Kimberly D. Ingram: Tracking spoilage bacteria in commercial poultry processing and refrigerated storage of poultry carcasses . In: International Journal of Food Microbiology . tape 91 , no. 2 , p. 155-165 , doi : 10.1016 / S0168-1605 (03) 00377-5 .