Dihydrotestosterone
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Androstanolone | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 19 H 30 O 2 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 290.44 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
178-183 ° C |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
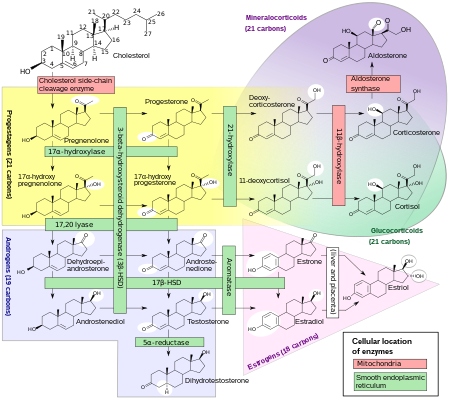
Dihydrotestosterone (DHT), more precisely 5 α -dihydrotestosterone , also androstanolone ( INN ), is a biologically active metabolite of the hormone testosterone . It is a C-19 steroid and belongs to the androgens , a class of sex hormones .
physiology
DHT is produced in the body from testosterone by the enzyme 5 α -reductase . DHT is the most biologically active form of testosterone. In many organs, DHT is the actually effective androgen .
Testosterone itself is a prohormone for two hormones: DHT and estradiol . DHT is a pure androgen as it cannot be flavored to estradiol. Even if testosterone and DHT work through the same receptor, their effects are still different. While testosterone induces the differentiation of Wolff's ducts , dihydrotestosterone is responsible for the external virilization and for the growth and differentiation of the prostate . Testosterone reaches androgen-dependent cells via the bloodstream. Intracellularly the hormone binds either directly to the androgen receptor , or is represented by the 5 α metabolized reductase to the biologically more effective DHT, which then exerts its effect also on the androgen receptor. The hormone-receptor complex enters the cell nucleus and binds there to specific hormone response elements (HRE) in the promoter region of androgen-regulated genes. This complex controls the activity and the specific cell response of these genes.
In the female organism
In women, DHT is made from testosterone and androstenedione . Only about 1% of DHT circulates freely, while the vast majority is firmly bound to sex hormone binding globulin (SHBG). DHT is inactivated by reduction to 17-keto steroids and excreted in the urine. Female androgens (C-19 steroids) are produced in the theca cells (the outer layer of cells around a follicle) in the ovaries and areas of the adrenal cortex . The hormonally most effective androgen at the steroid receptor is DHT, less effective are dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS). The DHEA from the means of the enzyme 3 β hydroxysteroid dehydrogenase resulting weakly effective androstenedione also comes to about 90% of the theca cells, the remaining 10% is converted into the peripheral tissues from the DHEA. But androstenedione provides a precursor for the female testosterone - synthesis . This represents synthesis or release into the female gonads is the luteinizing hormone (LH) of the anterior pituitary ( Examples of hormonal regulation ) of the stimulation by the releasing hormone gonadotropin releasing hormone (GnRH ) regulated with feedback , but in the adrenal cortex by the adrenocorticotropin (ACTH) .
The androsterones formed in the (adult) female organism mostly serve as a precursor to estrogen synthesis (C-18 steroids). The sex hormone-binding globulin (SHBG) is the specific transport protein for sex hormones, whereby only the non-bound, "free" DHT shows its physiological effect. As some other biologically active molecules for binding sites on the SHBG compete to a pregnancy , but also thyroxine lead to a change in levels of about DHT and other androgens.
In the male organism
Small amounts of DHT are made directly in the testicles in men . Dihydrotestosterone is the active form of testosterone that is only formed in the target cells. The development and function of the prostate and vesicle glands , body hair of the male type, beard growth , the function of the sebum glands , but also the decrease in head hair with genetic disposition are processes that are controlled by DHT. If the conversion of testosterone to dihydrotestosterone (DHT), which is necessary for the masculinization of the external genital system , is disrupted, for example in the case of a 5α-reductase-2 deficiency , this leads to various forms of genital malformations in genotypic male individuals. DHT in particular enables male development of the external genitalia, but also of the prostate . From the urogenital sinus arise the penis and the scrotum , into which the testicles, which enlarge under the influence of DHT, descend.
Function and side effects
Function and side effects of DHT correspond to those of its prohormone testosterone.
Diagnosis
The diagnosis of DHT is given for the following indications:
- Assessment and follow-up of pseudo-hermaphroditism
- Suspected genetic 5 α -reductase deficiency
- Therapy control of 5 α- reductase inhibitors ( finasteride , dutasteride ), for example in the treatment of benign prostatic hyperplasia (BPH) or prostate cancer
- Androgenization symptoms in women, such as hirsutism , virilization , alopecia
Low levels of DHT
For example, low DHT levels can be found in:
- Pseudohermaphroditism masculinus
- 5α-reductase deficiency
- Klinefelter Syndrome
- Primary and secondary hypogonadism
- Erectile dysfunction (impotence)
- Cirrhosis of the liver
- Estrogen therapy
- Therapy with 5α-reductase inhibitors such as finasteride and dutasteride
Elevated DHT levels
High DHT levels can be present in:
- Chronic anovulation syndromes, such as polycystic ovary syndrome
- Hirsutism
- Precocious puberty
- Congenital adrenal cortex - hyperplasia
- Adrenal cortex tumors, testicular tumors , ovarian tumors
In young men, DHT levels are around 10% of total testosterone levels. An increased testosterone / DHT quotient after HCG stimulation ( HCG test , Leydig cell function test) indicates a 5α-reductase deficiency.
Prostatic hyperplasia
DHT stimulates the growth of the prostate. If the function of DHT is inhibited, this leads to the size of the prostate. This form of therapy is often used for benign (benign) prostatic hyperplasia (BPH). For this purpose, 5α-reductase inhibitors with the active ingredients finasteride or dutasteride are taken orally.
Androgenetic alopecia ("hair loss")
Androgenetic alopecia (AGA), also known as hereditary hair loss, is widespread in men. It describes the genetically determined sensitivity of the hair roots to the hormone DHT. The sensitivity of the hair roots only affects the hair on the top of the head, where the hair follicles are damaged and weakened by DHT.
doping
The percentage of dihydrotestosterone users in bodybuilding has increased in recent years. More and more bodybuilders are using DHT to bring their androgen levels way beyond the natural limit. What causes great muscle growth on the one hand also has various side effects. Cardiac arrhythmias, liver and kidney tumors , arteriosclerosis and gynecomastia (swelling of the mammary glands in men) are just some of the side effects that can occur when using DHT.
In female athletes, a significant increase in body hair ( hirsutism ) in the facial area is possible and steroid acne can occur. Furthermore, hair loss with baldness is favored, a (permanent) deep voice can set in and permanent clitoral enlargement ( clitoral hypertrophy ) occurs. Also menstrual disorders and breast size changes detected; In pregnant women, disturbances in embryonic and fetal development ( sex determination ) are to be expected.
literature
- O. Hiort et al .: Androgen Resistance Syndromes - Clinical and Molecular Basis. In: Deutsches Ärzteblatt . 96/1999, SA-686 / B-560 / C-527.
- A. Kjellman et al .: Is dihydrotestosteron a prognostic factor among men with screening-detected prostate cancer? In: European Urology Supplements. 5/2006, p. 319.
- R. Paus: Therapeutic strategies for treating hair loss. In: Drug Discovery Today : Therapeutic Strategies. 3/2006, pp. 101-110.
- Julianne Imperato-Mcginley, Vivian Sobel, Yuan-Shan Zhu: Fetal hormones and sexual differentiation. In: Obstetrics and Gynecology Clinics of North America. Volume 31, No. 4, January 2005, pp. 837-856, doi: 10.1016 / j.ogc.2004.08.005 ( full text ).
Web links
- Dihydrotestosterone Compound - C03917 in the KEGG
Individual evidence
- ↑ a b c data sheet 5α-Androstan-17β-ol-3-one from Sigma-Aldrich , accessed on October 22, 2016 ( PDF ).
- ↑ a b c d e f g h i Endokrinologikum.com: Dihydrotestosteron ( Memento of the original from October 9, 2007 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. , accessed August 7, 2007.
- ↑ a b Genetics Center: Prostate Carcinoma Risk Analysis ( Memento of October 20, 2011 in the Internet Archive ), accessed on August 8, 2007.
- ↑ Thomas Weyerstahl, Manfred Stauber: Dual series gynecology and obstetrics. Georg Thieme Verlag, Stuttgart 2013, ISBN 3-13-152604-1 , pp. 100-102.
- ↑ Julianne Imperato-McGinley, Yuan-Shan Zhu: Androgens and male physiology — The syndrome of 5 alpha-reductase-2 deficiency. In: Molecular and Cellular Endocrinology. Volume 19, No. 1, December 2002, pp. 51-59, doi: 10.1016 / S0303-7207 (02) 00368-4 ( researchgate.net full text).
- ↑ Hey-Joo Kang, Julianne Imperato-McGinley, Yuan-Shan Zhu, Zev Rosenwaks: The effect of 5α-reductase-2 deficiency on human fertility. In: Fertility and sterility. Volume 101, No. 2, January 2014, pp. 310-316, doi: 10.1016 / j.fertnstert.2013.11.128 ( fertstert.org full text).
- ↑ bnw-natur.com: Hormonal Hair Loss ( Memento from August 13, 2007 in the Internet Archive ), accessed on August 8, 2007.
- ^ RD Goldman: Drug-induced gynecomastia in children and adolescents. In: Canadian family physician Medecin de famille canadien. Volume 56, Number 4, April 2010, pp. 344-345, PMID 20393092 , PMC 2860825 (free full text).