CRISPR
CRISPR ( C lustered R egularly I nterspaced S hort P alindromic R epeats) are portions to repetitive DNA ( repeats ), which in the genome of many bacteria and archaea occur. They serve a mechanism, the CRISPR / Cas system, which provides resistance to the penetration of foreign genes by viruses or plasmids , and are therefore part of the immune system equivalent of many prokaryotes . This system forms the basis of the genetic engineering CRISPR / Cas method for generating genetically modified organisms .
Discovery and Properties
The existence of repetitive stretches of DNA, now known as CRISPR, was discovered in 1987 in the bacterial strain Escherichia coli K12 by Yoshizumi Ishino and colleagues. They identified a repeating sequence of 29 nucleotides that were interrupted by the variable regions of 32 nucleotides. In 1993, similar regions were discovered on the DNA of Mycobacterium tuberculosis and referred to as "Direct Variable Repeats" (DVR). In 1995, these sequences were also discovered in the marine bacteria Haloferax volcanii and Haloferax mediterranei by the Spanish microbiologist Francisco Mojica , who called them " Tandem Repeats ”(TREPs). The Mojica working group identified other bacteria and archaea with corresponding sequences and chose a new name for these identical repetitions as “Short Regularly Spaced Repeats” (SRSR). In the literature, other names were added that also referred to these sequences, such as "spacer interspersed and direct repeats" (SPIDRs) and "long clustered tandem repeats" (LCTRs). In 2002, Jansen and colleagues first used the term “Clustered Regularly Interspaced Short Palindromic Repeats”, or CRISPR for short. It became known that similar structures exist in the genome of many different prokaryotes, and a group of genes was discovered that were close to the CRISPR gene locus in all of the organisms examined and were therefore called cas genes (CRISPR-associated). Jansen and colleagues identified four different Cas core sequences (Cas1 to Cas4); by 2005, Haft and colleagues had identified a total of 41 corresponding genes and two further Cas core sequences (Cas5 and Cas6) and a total of eight subtypes of CRISPR / Cas- Systems.
Today it is known that the genome of around 45% of the bacteria sequenced so far and 83% of the archaea contain at least one CRISPR structure.
Pathogens of the species Francisella use the CRISPR-Cas system for immune evasion . In Neisseria meningitidis and Campylobacter jejuni , the system is a pathogenicity factor with a previously unknown mechanism.
structure
The CRISPR locus consists essentially of two main components: the cas genes containing cas - Operon and the CRISPR array, which is composed of a leader composed (also called repeat-spacer-array) sequence, and a repeat spacer sequence.
Repeat spacer sequence
The individual sequences of the repeating basic motif ( repeats ) have a length that varies between 23 and 47 bp . The repeats alternate with spacers that are 21 to 72 bp in length. While the repeating sequence is retained within a CRISPR structure, the sequence of the CRISPR varies greatly in different microorganisms. The sequence of CRISPR repeats of the bacteria is usually palindromic (i.e. mirror-inverted complementary), which results in a stable secondary structure of the associated RNA , whereas most repeats of the archaea are not palindromic.
The sequences of the spacer sections vary widely, both within a CRISPR structure and in different prokaryotes . In 2005 it was discovered that the spacer sequences are identical to foreign DNA from bacteriophages and plasmids. This led to the hypothesis that the function of CRISPR is to defend the organism against foreign DNA.
cas operon
The cas operon is also part of the CRISPR gene locus . The cas operon contains cas genes and the coding proteins that are necessary for the adaptive immune response, e.g. B. helicases , nucleases , but also proteins with properties for RNA binding. cas -Gene can be divided into two modules: the effector and the adaptation module . An effector module is a group of cas genes that is used to identify genetic material . The adaptation module also contains cas genes and, with the help of effector proteins, contributes to the selection of protospacers that can be integrated into the bacterial genome.
leader sequence
In the vicinity of the repeat spacer sequence there is a so-called leader sequence (not to be confused with the leader sequence of the mRNA). The leader sequence is an adenine and thymine- rich sequence with a length of 100-500 bp. As with the repeats, leader sequences are approx. 80% identical within a genome, but show significant differences within different organisms. As a non-coding sequence, it can be divided into two areas: a core leader and an extended leader . The core leader is preserved in several organisms and with a length of 20–300 bp is usually shorter than the extended leader . In addition, the core leader has a promoter element to which regulatory proteins can bind in order to be able to control gene expression , more precisely the initiation of CRISPR transcription , and spacer acquisition.
The extended leader with a length of 50-500 bp longer than the core leader and also contains in the CRISPR-distant regions of conserved sequences, presumably through gene duplication have been executed. The functions of the extended leader are currently unknown. The extended leader probably has no important functions.
Immunity through CRISPR
The immunity through CRISPR takes place in three steps, whereby the last two steps are different for the respective CRISPR / Cas system types.
1. Adaptation: In 2007, Barrangou et al. Showed that bacteria that are infected with phages can integrate parts of the foreign DNA as spacers in the CRISPR regions of their genome and thereby develop immunity against the phages . They also showed that spacer sequences that are artificially inserted into the CRISPR regions of bacteria make them resistant to the associated phages. If the spacer sequences are cut out again, the resistance is also eliminated. It was also shown that the cas genes play an essential role in phage defense: the inactivation of some cas genes (cas1) prevents phage defense despite the presence of spacers. The activity of other cas genes (cas7) is necessary for the integration of new spacers in the CRISPR sequence.
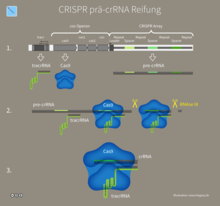
2. crRNA transcription and processing: The CRISPR gene locus is transcribed to pre-crRNA and then processed to mature crRNA.
3. Interference: The mature crRNA is associated with a Cas protein or a Cas protein complex and this leads to the formation of an interference complex. With the CRISPR / Cas system types I and II, interaction of the interference complex with the PAM sequence motif of the phage DNA leads to the degradation of the DNA with the help of Cas3 for type I and Cas9 for type II, whereas type III does not require PAM and RNA can be broken down as well as DNA.
In the course of coevolution , anti-CRISPR proteins were developed by bacteriophages to inhibit the immune system.
mechanism
Despite great advances in recent years, the mechanism by which the CRISPR / Cas system provides immunity to prokaryotes is not yet fully understood. It is assumed that in the immunization process the exogenous DNA is recognized by a Cas protein complex and integrated into the CRISPR areas as a new spacer. How these processes work in detail is not yet fully understood.
adaptation
CRISPR / Cas systems are able to modify the genome of bacteria and archaea by integrating foreign DNA sequences, so-called spacers, between the repeats of the CRISPR array. This process is known as adaptation or spacer acquisition. The adaptation can be divided into two phases:
- Capture of spacer sequences of foreign DNA (so-called protospacers),
- Spacer integration.
The mechanism of adaptation has, with a few exceptions, been studied in detail in the E. coli CRISPR / Cas system type I (also known as the CRISPR / Cas system type IE). The main actors of the adaptation are encoded by the genes cas1 and cas2 , which are conserved in different CRISPR / Cas system types.
The first phase of adaptation, the capture of spacer sequences from the foreign DNA, can take place in two modes with the CRISPR / Cas system type I: naive or primed . In naive adaptation , only the proteins Cas1 and Cas2 are needed to capture spacers without prejudice, whereas primed adaptation depends on existing spacers ( priming spacers ) and a pre-selection is made as to which spacers are integrated into the genome. In addition to the Cas1 and Cas2 proteins, a protein complex made up of Cas proteins (interference complex type I, Cascade ) and the Cas3 nuclease are also required for this. Other types of CRISPR / Cas systems encode additional proteins for adaptation.
The mechanism of primed adaptation begins with the binding of the crRNA-bound protein complex Cascade ( CRISPR-associated complex for antiviral defense ) to the Protospacer Adjacent Motif (PAM) of the invading DNA by means of a combination of facilitated 1D diffusion (sliding along the DNA) and 3D diffusion ( hopping ). After the DNA has been bent and untwisted by Cascade, an R-loop is formed through complementary base pairing of the crRNA and the Cascade-bound DNA strand . When the Protospacer is completely unwound by Cascade, the R-Loop is completely formed. The complete formation of the R-loop leads to a change in the conformation of Cascade and thus causes Cas3 to bind to Cascade. In addition, the complete formation of the R-loop triggers a bulge on the unbound strand and thus enables the cut through Cas3 at this bulge. The single-stranded fragments generated by Cas3 are then processed into single-stranded protospacers by the Cas1-Cas2 complex. After processing into a single-stranded protospacer, it is converted into a complete or partially double-stranded protospacer, so that integration into the CRISPR array is possible. Even after the last step of immunity through CRISPR, the interference, the capture of spacer sequences is possible. The fragments of the degraded DNA are converted into protospacers by the enzyme RecBCD or other nucleases and integrated into the CRISPR array with the help of the Cas1-Cas2 complex (naive adaptation).
The spacer integration does not occur randomly in the CRISPR array, but is polarized, i.e. H. that spacers are integrated at a specific point in the CRISPR array, more precisely in the vicinity of the leader sequence. This mechanism ensures that new spacers are always integrated in the vicinity of the leader sequence and the chronological integration of the spacers optimizes the adaptive immune response to the most recent viral infections. With the CRISPR / Cas system type I, the protein integration host factor (IHF) is required, which can bind to the leader sequence. This bends the leader sequence by about 120 ° and creates a binding site for the Cas1-Cas2 complex, so that the complex is located in the vicinity of the repeat that is located closest to the leader sequence. As a result, the leader- repeat boundary becomes the place of spacer integration. With the CRISPR / Cas system type II, the spacer integration is also polarized, but without the use of additional proteins. The α-helix of Cas1 of the Cas1-Cas2 complex type II binds to the small groove of the leader sequence, which is also known as the leader-anchoring sequence (LAS). Due to the flexibility of the LAS-interacting domain of Cas1, the spacer integration does not necessarily have to take place at the leader- repeat boundary, but can also take place at a spacer-repeat boundary. In the case of a mutated LAS, this can lead to ectopic spacer integration, with spacers being integrated in the middle of the CRISPR array.
In E. coli , the spacer integration takes place through two transesterifications , the first transesterification taking place through the nucleophilic attack of the hydroxyl group at the 3 'end of one strand of the protospacer at the leader- repeat border and thereby leading to the formation of a half-site - Integration intermediary leads. The first transesterification creates an inflection of the repeat that enables a second transesterification. The transition to the fully integrated spacer, the full-site product, takes place through a second transesterification, with the nucleophilic attack of the hydroxyl group at the 3 'end of the opposite strand of the protospacer in the vicinity of the repeat-spacer boundary. The second transesterification is regulated by a so-called ruler mechanism. In E. coli , the repeat contains two inverse repeats (IR) that code for structural motifs and serve as anchors for so-called “molecular rulers”. These molecular rulers ensure that the second nucleophilic attack only takes place in the vicinity of the repeat-spacer boundary and that the length of the repeat is maintained after spacer integration and repeat duplication. The DNA gaps created after the transesterifications are closed by various DNA repair mechanisms , including homology-directed repair (HDR), non-homologous end joining (NHEJ) and microhomology-mediated end joining (MMEJ). After the spacer integration had taken place, the repeat adjoining the leader sequence was duplicated with the same length.
crRNA transcription and processing
The biogenesis of a mature CRISPR-RNA (crRNA) can take place in three steps and, with the help of its partially unique spacer sequence, leads one or more Cas proteins to the penetrating nucleic acid , which is used for possible degradation of the genetic material after sequence-specific RNA recognition.
- Transcription of a long primary transcript, the precursor crRNA (pre-crRNA), by a promoter located within the leader sequence.
- Primary cleavage of the pre-crRNA at specific sites to generate crRNA with an entire spacer sequence with partial repeat sequences.
- In some cases, an additional secondary cleavage is required to generate an active mature crRNA.
In the CRISPR / Cas systems I and III, a specific endoribonuclease of the Cas6 family or, alternatively, Cas5d for type IC, is required which, alone or in complex with other Cas proteins, cleaves the pre-crRNA within the repeat regions. In type II, a tracrRNA transactivates the cleavage of the pre-crRNA within the repeat regions by endoribonuclease III ( RNase III ) in the presence of Cas9.
With the CRISPR / Cas system type I, the processing of the pre-crRNA is catalyzed by endoribonucleases of the metal-independent Cas6 family (or alternatively with type IC by Cas5d), which place the repeat sequence at conserved positions, typically 8 nt upstream ("upstream") , in the direction of the 5 'end) from the repeat-spacer boundary, splits. While only one maturation step is required for types IC, IE and IF, a second maturation step is required for types IA, IB and ID, the components and mechanism of which are currently unknown. The palindromic repeats of the pre-crRNA of the types IC, ID, IE and IF have hairpin structures that expose the cleavage sites for the catalytic domain of the respective endoribonuclease. After cleavage, the hairpin structures remain associated with the respective endoribonuclease and the subunits of Cascade bind to the sequence at the 5 'end and to the spacer, which are used to recognize genetic material.
In the CRISPR / Cas system type II, after transcription of the CRISPR array and of tracrRNA, base pairing of the anti-repeat of tracrRNA with the repeat of the pre-crRNA takes place and the formation of the tracrRNA: pre-crRNA duplex, which is stabilized by Cas9 . In addition, the duplex formation leads to the recruitment of RNase III and thus to the co-processing of the duplex. This is followed by the second maturation step , with trimming by an exonuclease and / or cleavage by an endoribonuclease. Type II-C represents an alternative synthesis route for a mature crRNA. The promoters are located within the repeats of the CRISPR array and a short pre-crRNA transcript can be formed so that cleavage by RNase III is no longer necessary . The mature duplex is complexed with Cas9 and forms a type II interference complex that can recognize and cleave double-stranded DNA (dsDNA).
In the CRISPR / Cas system type III, the cleavage of the pre-crRNA within the repeats takes place by Cas6 and thus generates crRNA intermediates that each have a partial sequence of the repeats of the pre-crRNA at their 5 'and 3' ends ( 1X intermediate). Thereafter, the 1X intermediate is complexed with the Csm complex in III-A and with the Cmr complex in III-B. The second maturation step then takes place by trimming at the 3 'end using nucleases, which have not yet been identified, to form the mature crRNA.
interference
The after processing of the pre-crRNA to mature crRNA, which contain the integrated viral spacer sequences, associate with a CRISPR ribonucleoprotein complex (crRNP) and form an interference complex (also known as CRISPR surveillance complex) with the after a further infection, the viral DNA or RNA can be degraded sequence-specifically. The interference mechanism in all CRISPR / Cas system types is characterized by certain key proteins: Cas 3 (type I), Cas 9 (type II) and Cas10 (type III) and differ mainly in the structure of the crRNP complex (crRNP assembly ) and in the mechanism of degradation of genetic material. All crRNP complexes in type I are called cascades, whereas in type II the protein Cas9 as a single protein is responsible for the cleavage of the nucleic acid. In type III, the crRNP complexes Csm (type III-A) and Cmr (type III-B) are responsible for the interference.
With the CRISPR / Cas system type I, the interference occurs in five steps:
- Cascade assembly
- PAM detection and retention
- R-loop formation
- Cas3 recruitment
- DNA degradation
After processing the pre-crRNA, the mature crRNA of E. coli consists of a 5 ' handle (8 nt) with a hydroxyl group, a spacer sequence (32 nt) and a hairpin structure at the 3' end (21 nt) a 2'-3'-cyclic phosphate end, with Cas6e remaining associated with the hairpin structure after processing. After cleavage of the mature crRNA, the cascade assembly takes place, the first step being termini capping . Cas5 binds to the 5'- handle and thus initially creates a hook-like structure of the crRNA. In addition, six copies of the Cas7 protein bind to the spacer sequence, resulting in the so-called Cas7 backbone . What is special is that the structures of Cas5 and Cas7 have a so-called conserved “palm-thumb domain”, which contribute to the interweaving of the Cas7 backbone. The “thumb” (a β-hairpin structure) of either Cas5e or of each of the six Cas7 subunits (Cas7.1 – Cas7.6) kinks the crRNA on the 5′- handle at a certain position and at 6-nt intervals within the spacer sequence and ensures that the kinked nucleotides adopt a deformed configuration and are no longer suitable for base pairing with the target DNA. In contrast, the adjacent 5-nt sequences protrude at every bend and retain their discontinuous A-DNA -like shape, so that these sequences are suitable for base pairing with the target DNA. Two other proteins, Cse1 (large subunit) and the Cse2 dimer (small subunits), then bind to the Cas7 subunits by means of protein-protein interaction . Both proteins are involved in DNA binding, with the large subunit also contributing to target selection. This ensures that the interference complex searches the cell for potential target DNA at all times. After the final assembly, Cascade is often described as a seahorse- like structure.
Now, with the help of Cascade, the search for the target DNA takes place, with the L1 loop of Cse1 being responsible for PAM identification. In type IE, after PAM identification, the double-stranded viral DNA enters the gap between Cas7.5 and Cas7.6 and is then passed on to the large subunit (Cse1), which, however, mainly has non-specific interactions with the target DNA. The PAM recognition by the L1 loop of Cse1 causes a destabilization of the double-stranded DNA, so that the base pairing between the 7 nt long seed region of the PAM-bordering DNA protospacer sequence with the crRNA can take place. The subsequent formation of an R-loop with complete base pairing of the crRNA spacer with the viral protospacer takes place according to the same mechanism as when capturing spacer sequences. After complete R-loop formation, the conformation of the large and small subunits changes, so that interaction sites are created on the large subunit for the C -terminal domain (CTD) of Cas3. Recruiting Cas3 at the bifurcation opens the channel for the double-stranded DNA by dissociating the CTD. After the dsDNA has accumulated in the channel, the channel is closed by repositioning the CTD and the unbound strand of the double-stranded DNA is stored in the HD nuclease domain of Cas3, where the cut takes place. The cut is made approximately 11–15 nt downstream (“downstream”, towards the 3 'end) of the PAM with the help of two catalytic transition metal ions. The conformational change of Cas3 in the helica part triggered by the cut (consisting of the RecA-like domain ( RecA ) and the RecA-like domain 2 ( RecA2 )) causes ATP binding and hydrolysis , the energy released for unwinding the dsDNA in 3 ′ → 5 ′ direction is used. The unwinding takes place on a hairpin structure of RecA2. By moving the helica part, this triggers a shift of the HD domain to new substrates for further exonucleolytic degradation. The single-stranded DNA (ssDNA) formed after degradation are also exonucleolytically degraded by Cas3. Thus, the target DNA can be effectively removed from the cell and Cascade can be recycled for further PAM recognition.
With the CRISPR / Cas system type II, the interference occurs in four steps:
- Formation of the active type II CRISPR surveillance complex
- PAM detection and retention
- R-loop formation
- DNA degradation
Three independent studies on the structure of Cas9 by S. pyogenes show that Cas9 consists of two lobes that together adopt a crescent moon conformation. The REC lobe (English recognition lobe ) consists of a long α-helix (bridge helix), a Rec2 domain and a Rec1 domain to recognize the tracrRNA: crRNA duplex. The NUC lobe (English nuclease lobe ) consists of two nuclease domains for DNA cleavage, which are called HNH (named after characteristic histidine and asparagine residues ) and RuvC (named after an E. coli protein that is attached to the DNA participates repair) are known, and an additional C -terminal topoisomerase - homology domain (CTD), which is necessary to facilitate the PAM recognition.
The activation of Cas9 by binding the duplex to Rec1 before the interference triggered a conformational change of HNH, which led to the change in position of the REC lobe and the formation of a central positively charged channel for the invading DNA. The active type II CRISPR surveillance complex formed after co-processing the duplex is now ready to search for a viral DNA with a PAM sequence. After the PAM binding, local melting of the DNA occurs. Here unpaired are nucleic bases , so-called molten bubbles (engl. Melted bubbles ) formed nt to R-loop formation at a PAM proximal 8-12 long seed contribute sequence of the DNA. Each nuclease domain then cleaved a DNA strand in the presence of Mg 2+ ions, the HNH domain cleaving the target DNA strand hybridized to the crRNA and the RuvC domain cleaving the unhybridized DNA strand. The resulting cut, which takes place about 3 nt up the strand from the PAM, leads to the formation of double strand breaks with blunt ends (English for "smooth end"). Thereafter, Cas9 remains firmly associated with the blunt ends of the viral DNA.
In type III CRISPR / Cas systems, the interference complex recognizes the resulting RNA transcript, which is complementary to the sequence of the crRNA spacer, and degrades both the transcript and the DNA from which the transcript arose. This process is known as transcription-dependent DNA interference. The interference complex has three enzymatic activities:
- crRNA-directed endoribonuclease activity against the target RNA by Csm3 (type III-A) or Cmr4 (type III-B)
- Target RNA-stimulated DNase activity through the HD domain of Cas10 (Csm1 in types III-A and III-D or Cmr2 in types III-B and III-C)
- Target-RNA-stimulated cOA (cyclic oligoadenylate) synthetase activity through the "palm domain" of Cas10 (Csm1 for types III-A and III-D or Cmr2 for types III-B and III-C)
In bacteria, the crRNA-controlled complexes Csm (type III-A) or Cmr (type III-B) are brought to the RNA transcript, which triggers the cleavage of the transcript by the subunits Csm3 or Cmr4 and at the same time the DNase activity of Csm1 or Cmr2 activated for coupled degradation of ssDNA in the transcription bubble. The “palm domain”, more precisely the cyclase domain, of Csm1 or Cmr2 can produce cOA from ATP when the RNA transcript is bound. cOA in turn binds and activates the ribonuclease Csm6 (type III-A) or Csx1 (type III-B, III-C and III-D) to increase their ribonuclease activity in order to degrade RNA transcripts and thus form an additional interference Mechanism.
Effects
Through the CRISPR / Cas mechanism, bacteria can acquire immunity to certain phages and pass on the acquired immunity, as they integrate a virus-specific spacer into their genome and thus pass it on during replication . For this reason, the provocative thesis was expressed that the CRISPR-Cas system was the first really Lamarckist inheritance mechanism.
Applications
There are several suggestions for using CRISPR biotechnologically :
- Artificial immunization against phages by adding suitable spacers to industrially important bacteria, e.g. B. in the dairy or wine industry ,
- Knockdown of endogenous genes by transformation with a plasmid containing a CRISPR region with crRNA that matches the gene to be shut down,
- Multiplex Genome Editing allows the simultaneous mutation of different target sequences, which shortens the production time of transgenic animals such as mice from up to two years to a few weeks,
- Differentiation of different bacterial strains by comparing the spacer regions ( spoligotyping ),
- Gene therapy ,
- Fluorescent marking .
literature
- Martin Jinek , Krzysztof Chylinski, Ines Fonfara, Michael Hauer, Jennifer Doudna , Emmanuelle Charpentier : A Programmable Dual-RNA – Guided DNA Endonuclease in Adaptive Bacterial Immunity (PDF). In: Science , Vol. 337, No. 6096, August 17, 2012, pp. 816 ff. ISSN 0036-8075 . (English)
Individual evidence
- ↑ Y. Ishino, H. Shinagawa K. Makino M. Amemura A. Nakata A: Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. Journal of Bacteriology 169: 5429-5433 (1987)
- ^ PM Groenen, AE Bunschoten, D. van Soolingen, JD van Embden: Nature of DNA polymorphism in the direct repeat cluster of Mycobacterium tuberculosis; application for strain differentiation by a novel typing method. Molecular Microbiolology 10, 1993; Pp. 1057-1065.
- ↑ FJ Mojica, C. Ferrer, G. Juez, F. Rodriguez-Valera: Long stretches of short tandem repeats are present in the largest replicons of the Archaea Haloferax mediterranei and Haloferax volcanii and could be involved in replicon partitioning. Molecular Microbiology 17, 1995; Pp. 85-93.
- ^ FJ Mojica, C. Diez-Villasenor, E. Soria, G. Juez: Biological significance of a family of regularly spaced repeats in the genomes of Archaea, Bacteria and mitochondria. Molecular Microbiology 36, 2000; Pp. 244-246.
- ↑ a b Sinan Al-Attar, Edze R. Westra, John van der Oos, Stan JJ Brouns: Clustered regularly interspaced short palindromic repeats (CRISPRs): the hallmark of an ingenious antiviral defense mechanism in prokaryotes. Biological Chemistry 392, 2011; Pp. 277-289. doi: 10.1515 / BC.2011.042 , full text
- ↑ R. Jansen, JD Embden, W. Gaastra, LM Schouls: Identification of genes that are associated with DNA repeats in prokaryotes. Mol Microbiol 43: 1565-1575 (2002)
- ↑ DH Haft, J. Selengut, EF Mongodin, KE Nelson: A guild of 45 CRISPR-associated (Cas) protein families and multiple CRISPR / Cas subtypes exist in prokaryotic genomes. PLoS Comput. Biol. 1, 2005; e60.
- ↑ 125/150 Archaea, 1126/2480 Bacteria CRISPRdb , as of January 19, 2013
- ↑ a b T. R. Sampson, DS Weiss: Alternative roles for CRISPR / Cas systems in bacterial pathogenesis. In: PLoS pathogens. Volume 9, number 10, 2013, p. E1003621, ISSN 1553-7374 . doi: 10.1371 / journal.ppat.1003621 . PMID 24146613 . PMC 3798603 (free full text).
- ↑ a b c A. Horvath, R. Barrangou: CRISPR / Cas, the Immune System of Bacteria and Archaea. Science 327, p. 167 (2010)
- ↑ LA Marraffini, EJ Sontheimer: CRISPR interference: RNA-directed adaptive immunity in bacteria and archaea. In: Nature Reviews Genetics . Volume 11, number 3, March 2010, pp. 181-190, doi : 10.1038 / nrg2749 , PMID 20125085 , PMC 2928866 (free full text) (review).
- ↑ a b c d E. Charpentier, H. Richter, J. van der Oost, MF White: Biogenesis pathways of RNA guides in archaeal and bacterial CRISPR-Cas adaptive immunity. In: FEMS microbiology reviews. Volume 39, number 3, May 2015, pp. 428-441, doi : 10.1093 / femsre / fuv023 , PMID 25994611 , PMC 5965381 (free full text) (review).
- ↑ a b c d e CRISPR Locus. In: Sino Biological. Retrieved January 11, 2020 .
- ↑ FJ Mojica, C. Diez-Villasenor, J. Garcia-Martinez, E. Soria: Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. Journal of Molecular Evolution 60: 174-182 (2005).
- ↑ C. Pourcel, G. Salvignol, G. Vergnaud: CRISPR elements in Yersinia pestis acquire new repeats by preferential uptake of bacteriophage DNA, and provide additional tools for evolutionary studies. Microbiology 151: 653-663 (2005).
- ↑ A. Bolotin, B. Quinquis, A. Sorokin, SD Ehrlich: Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology 151: 2551-2561 (2005).
- ↑ Donghyun Ka, Dong Man Jang, Byung Woo Han, Euiyoung Bae: Molecular organization of the type II-A CRISPR adaptation module and its interaction with Cas9 via Csn2. In: Nucleic Acids Research. 46, 2018, p. 9805, doi : 10.1093 / nar / gky702 .
- ^ Eugene V. Koonin: CRISPR: a new principle of genome engineering linked to conceptual shifts in evolutionary biology. In: Biology & Philosophy. Volume 34, 2019, doi : 10.1007 / s10539-018-9658-7 .
- ↑ Omer S. Alkhnbashi, Shiraz A. Shah, Roger A. Garrett, Sita J. Saunders, Fabrizio Costa, Rolf Ofen: Characterizing leader sequences of CRISPR loci. In: Bioinformatics. Volume 32, Number 17, September 1, 2016, pp. I576 – i585, doi : 10.1093 / bioinformatics / btw454 .
- ^ A b R. Barrangou, C. Fremaux, H. Deveau, M. Richards, P. Boyaval, S. Moineau, DA Romero, A. Horvath: CRISPR Provides Acquired Resistance Against Viruses in Prokaryotes. Science 315, 1709-1712 (2007)
- ↑ Jennifer Doudna , Emmanuelle Charpentier .: The new frontier of genome engineering with CRISPR-Cas9. Science 346 (6213): 1077 (2014), PMID 25430774 , doi: 10.1126 / science.1258096 .
- ↑ CRISPR Spacer Acquisition. In: Sino Biological. Retrieved January 16, 2020 .
- ↑ a b Kirill A. Datsenko, Ksenia Pougach, Anton Tikhonov, Barry L. Wanner, Konstantin Severinov, Ekaterina Semenova: Molecular memory of prior infections activates the CRISPR / Cas adaptive bacterial immunity system. In: Nature Communications. Volume 3, 2012, doi : 10.1038 / ncomms1937 .
- ↑ Daan C. Swarts, Cas Mosterd, Mark WJ van Passel, Stan JJ Brouns, Igor Mokrousov: CRISPR Interference Directs Strand Specific Spacer Acquisition. In: PLoS ONE. Volume 7, 2012, p. E35888, doi : 10.1371 / journal.pone.0035888 .
- ↑ Ido Yosef, Moran G. Goren, Udi Qimron: Proteins and DNA elements essential for the CRISPR adaptation process in Escherichia coli. In: Nucleic Acids Research. Volume 40, 2012, p. 5569, doi : 10.1093 / nar / gks216 .
- ↑ PC Fineran, MJH Gerritzen, M. Suarez-Diez, T. Kunne, J. Boekhorst, Sacha AFT van Hijum, Raymond HJ Staals, Stan JJ Brouns: Degenerate target sites mediate rapid primed CRISPR adaptation. In: Proceedings of the National Academy of Sciences. Volume 111, number 16, 2014, pp. E1629 – E1638, doi : 10.1073 / pnas.1400071111 .
- ↑ a b Kaylee E. Dillard, Maxwell W. Brown, Nicole V. Johnson, Yibei Xiao, Adam Dolan, Erik Hernandez, Samuel D. Dahlhauser, Yoori Kim, Logan R. Myler, Eric V. Anslyn, Ailong Ke, Ilya J Finkelstein: Assembly and Translocation of a CRISPR-Cas Primed Acquisition Complex. In: Cell. Volume 175, 2018, p. 934, doi : 10.1016 / j.cell.2018.09.039 .
- ↑ a b c d S.JJ Brouns, MM Jore, M. Lundgren, ER Westra, RJH Slijkhuis, APL Snijders, MJ Dickman, KS Makarova, EV Koonin, J. van der Oost: Small CRISPR RNAs Guide Antiviral Defense in Prokaryotes . Science 321: 960 (2008)
- ↑ Yibei Xiao, Min Luo, Robert P. Hayes, Jonathan Kim, Sherwin Ng, Fang Ding, Maofu Liao, Ailong Ke: Structure Basis for Directional R-loop Formation and Substrate Handover Mechanisms in Type I CRISPR-Cas System. In: Cell. Volume 170, 2017, p. 48, doi : 10.1016 / j.cell.2017.06.012 .
- ↑ Olga Musharova, Evgeny Klimuk, Kirill A. Datsenko, Anastasia Metlitskaya, Maria Logacheva, Ekaterina Semenova, Konstantin Severinov, Ekaterina Savitskaya: Spacer-length DNA intermediates are associated with Cas1 in cells undergoing primed CRISPR adaptation. In: Nucleic Acids Research. Volume 45, 2017, p. 3297, doi : 10.1093 / nar / gkx097 .
- ^ R. Sorek, CM Lawrence, B. Wiedenheft: CRISPR-mediated adaptive immune systems in bacteria and archaea. In: Annual review of biochemistry. Volume 82, 2013, pp. 237-266, doi : 10.1146 / annurev-biochem-072911-172315 , PMID 23495939 (review).
- ↑ KN Yoganand, R. Sivathanu, S. Nimkar, B. Anand: Asymmetric positioning of Cas1-2 complex and Integration Host Factor induced DNA bending guide the unidirectional homing of protospacer in CRISPR-Cas type IE system. In: Nucleic acids research. Volume 45, number 1, January 2017, pp. 367-381, doi : 10.1093 / nar / gkw1151 , PMID 27899566 , PMC 5224486 (free full text).
- Jump up ↑ Y. Xiao, S. Ng, KH Nam, A. Ke: How type II CRISPR-Cas establish immunity through Cas1-Cas2-mediated spacer integration. In: Nature . Volume 550, number 7674, 10 2017, pp. 137–141, doi : 10.1038 / nature24020 , PMID 28869593 , PMC 5832332 (free full text).
- ↑ J. McGinn, LA Marraffini: CRISPR-Cas system Optimize Their Immune Response by Specifying the site of spacer integration. In: Molecular cell. Volume 64, number 3, 11 2016, pp. 616–623, doi : 10.1016 / j.molcel.2016.08.038 , PMID 27618488 , PMC 5096952 (free full text).
- ↑ JK Nuñez, AS Lee, A. Engelman, JA Doudna: Integrase-mediated spacer acquisition during CRISPR-Cas adaptive immunity. In: Nature . Volume 519, number 7542, March 2015, pp. 193–198, doi : 10.1038 / nature14237 , PMID 25707795 , PMC 4359072 (free full text).
- ↑ a b Jenny G Kim, Sandra Garrett, Yunzhou Wei, Brenton R. Graveley, Michael P. Terns: CRISPR DNA elements controlling site-specific integration and proper spacer repeat length by a Type II CRISPR-Cas system. In: Nucleic Acids Research . Volume 47, 2019, p. 8632, doi : 10.1093 / nar / gkz677 .
- ^ MG Goren, S. Doron, R. Globus, G. Amitai, R. Sorek, U. Qimron: Repeat Size Determination by Two Molecular Rulers in the Type IE CRISPR Array. In: Cell Reports. Volume 16, number 11, September 2016, pp. 2811-2818, doi : 10.1016 / j.celrep.2016.08.043 , PMID 27626652 , PMC 5039180 (free full text).
- ^ SH Sternberg, H. Richter, E. Charpentier, U. Qimron: Adaptation in CRISPR-Cas Systems. In: Molecular cell. Volume 61, Number 6, March 2016, pp. 797-808, doi : 10.1016 / j.molcel.2016.01.030 , PMID 26949040 (review).
- ↑ a b J. Carte, R. Wang, H. Li, RM Terns, MP Terns: Cas6 is an endoribonuclease that generates guide RNAs for invader defense in prokaryotes. In: Genes & development. Volume 22, number 24, December 2008, pp. 3489-3496, doi : 10.1101 / gad.1742908 , PMID 19141480 , PMC 2607076 (free full text).
- ↑ a b c MM Jore, M. Lundgren, E. van Duijn, JB Bultema, ER Westra, SP Waghmare, B. Wiedenheft, U. Pul, R. Wurm, R. Wagner, MR Beijer, A. Barendregt, K. Zhou, AP Snijders, MJ Dickman, JA Doudna, EJ Boekema, AJ Heck, J. van der Oost, SJ Brouns: Structural basis for CRISPR RNA-guided DNA recognition by Cascade. In: Nature structural & molecular biology. Volume 18, number 5, May 2011, pp. 529-536, doi : 10.1038 / nsmb.2019 , PMID 21460843 .
- ↑ E. Deltcheva, K. Chylinski, CM Sharma, K. Gonzales, Y. Chao, ZA Pirzada, MR Eckert, J. Vogel, E. Charpentier: CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. In: Nature . Volume 471, number 7340, March 2011, pp. 602-607, doi : 10.1038 / nature09886 , PMID 21455174 , PMC 3070239 (free full text).
- ^ Y. Zhang, N. Heidrich, BJ Ampattu, CW Gunderson, HS Seifert, C. Schoen, J. Vogel, EJ Sontheimer: Processing-independent CRISPR RNAs limit natural transformation in Neisseria meningitidis. In: Molecular cell. Volume 50, number 4, May 2013, pp. 488-503, doi : 10.1016 / j.molcel.2013.05.001 , PMID 23706818 , PMC 3694421 (free full text).
- ↑ M. Jinek, K. Chylinski, I. Fonfara, M. Hauer, JA Doudna, E. Charpentier: A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. In: Science . Volume 337, number 6096, August 2012, pp. 816-821, doi : 10.1126 / science.1225829 , PMID 22745249 , PMC 6286148 (free full text).
- ↑ J. Carte, NT Pfister, MM Compton, RM Terns, MP Terns: Binding and cleavage of CRISPR RNA by Cas6. In: RNA. Volume 16, number 11, November 2010, pp. 2181-2188, doi : 10.1261 / rna.2230110 , PMID 20884784 , PMC 2957057 (free full text).
- ↑ KS Makarova, DH Haft, R. Barrangou, SJ Brouns, E. Charpentier, P. Horvath, S. Moineau, FJ Mojica, YI Wolf, AF Yakunin, J. van der Oost, EV Koonin: Evolution and classification of the CRISPR -Cas systems. In: Nature reviews. Microbiology. Volume 9, number 6, 06 2011, pp. 467-477, doi : 10.1038 / nrmicro2577 , PMID 21552286 , PMC 3380444 (free full text) (review).
- ↑ a b A. Plagens, H. Richter, E. Charpentier, L. Randau: DNA and RNA interference mechanisms by CRISPR-Cas surveillance complexes. In: FEMS microbiology reviews. Volume 39, number 3, May 2015, pp. 442-463, doi : 10.1093 / femsre / fuv019 , PMID 25934119 , PMC 5965380 (free full text) (review).
- ↑ RN Jackson, SM Golden, PB van Erp, J. Carter, ER Westra, SJ Brouns, J. van der Oost, TC Terwilliger, RJ Read, B. Wiedenheft: Structural biology. Crystal structure of the CRISPR RNA-guided surveillance complex from Escherichia coli. In: Science . Volume 345, number 6203, September 2014, pp. 1473–1479, doi : 10.1126 / science.1256328 , PMID 25103409 , PMC 4188430 (free full text).
- ↑ B. Wiedenheft, GC Lander, K. Zhou, MM Jore, SJ Brouns, J. van der Oost, JA Doudna, E. Nogales: Structures of the RNA-guided surveillance complex from a bacterial immune system. In: Nature . Volume 477, number 7365, September 2011, pp. 486-489, doi : 10.1038 / nature10402 , PMID 21938068 , PMC 4165517 (free full text).
- ↑ DG Sashital, B. Wieden book, YES Doudna: Mechanism of foreign DNA into a bacterial selection adaptive immune system. In: Molecular cell. Volume 46, number 5, June 2012, pp. 606-615, doi : 10.1016 / j.molcel.2012.03.020 , PMID 22521690 , PMC 3397241 (free full text).
- ^ ER Westra, PB van Erp, T. Künne, SP Wong, RH Staals, CL Seegers, S. Bollen, MM Jore, E. Semenova, K. Severinov, WM de Vos, RT Dame, R. de Vries, SJ Brouns , J. van der Oost: CRISPR immunity relies on the consecutive binding and degradation of negatively supercoiled invader DNA by Cascade and Cas3. In: Molecular cell. Volume 46, number 5, June 2012, pp. 595-605, doi : 10.1016 / j.molcel.2012.03.018 , PMID 22521689 , PMC 3372689 (free full text).
- ↑ a b B. Gong, M. Shin, J. Sun, CH Jung, EL Bolt, J. van der Oost, JS Kim: Molecular insights into DNA interference by CRISPR-associated nuclease-helicase Cas3. In: Proceedings of the National Academy of Sciences . Volume 111, number 46, November 2014, pp. 16359-16364, doi : 10.1073 / pnas.1410806111 , PMID 25368186 , PMC 4246338 (free full text).
- ^ A b Y. Huo, KH Nam, F. Ding, H. Lee, L. Wu, Y. Xiao, MD Farchione, S. Zhou, K. Rajashankar, I. Kurinov, R. Zhang, A. Ke: Structures of CRISPR Cas3 offer mechanistic insights into Cascade-activated DNA unwinding and degradation. In: Nature structural & molecular biology. Volume 21, number 9, September 2014, pp. 771-777, doi : 10.1038 / nsmb.2875 , PMID 25132177 , PMC 4156918 (free full text).
- ↑ a b S. Mulepati, S. Bailey: In vitro reconstitution of an Escherichia coli RNA-guided immune system reveals unidirectional, ATP-dependent degradation of DNA target. In: Journal of Biological Chemistry . Volume 288, number 31, August 2013, pp. 22184-22192, doi : 10.1074 / jbc.M113.472233 , PMID 23760266 , PMC 3829311 (free full text).
- ↑ a b T. Sinkunas, G. Gasiunas, C. Fremaux, R. Barrangou, P. Horvath, V. Siksnys: Cas3 is a single-stranded DNA nuclease and ATP-dependent helicase in the CRISPR / Cas immune system. In: The EMBO Journal . Volume 30, number 7, April 2011, pp. 1335-1342, doi : 10.1038 / emboj.2011.41 , PMID 21343909 , PMC 3094125 (free full text).
- ↑ M. Jinek, F. Jiang, DW Taylor, SH Sternberg, E. Kaya, E. Ma, C. Anders, M. Hauer, K. Zhou, S. Lin, M. Kaplan, AT Iavarone, E. Charpentier, E. Nogales, JA Doudna: Structures of Cas9 endonucleases reveal RNA-mediated conformational activation. In: Science . Volume 343, number 6176, March 2014, p. 1247997, doi : 10.1126 / science.1247997 , PMID 24505130 , PMC 4184034 (free full text).
- ↑ a b C. Anders, O. Niewoehner, A. Duerst, M. Jinek: Structural basis of PAM-dependent target DNA recognition by the Cas9 endonuclease. In: Nature . Volume 513, number 7519, September 2014, pp. 569-573, doi : 10.1038 / nature13579 , PMID 25079318 , PMC 4176945 (free full text).
- ↑ a b H. Nishimasu, FA Ran, PD Hsu, S. Konermann, SI Shehata, N. Dohmae, R. Ishitani, F. Zhang, O. Nureki: Crystal structure of Cas9 in complex with guide RNA and target DNA. In: Cell . Volume 156, number 5, February 2014, pp. 935-949, doi : 10.1016 / j.cell.2014.02.001 , PMID 24529477 , PMC 4139937 (free full text).
- ↑ a b Elitsa Y. Dimova, Thomas Kietzmann: Genome changes – CRISPR / Cas9 as method of choice or agony? In: BIOspectrum. 24, 2018, p. 702, doi : 10.1007 / s12268-018-0977-7 .
- ↑ a b S. H. Sternberg, S. Redding, M. Jinek, EC Greene, JA Doudna: DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. In: Nature . Volume 507, number 7490, March 2014, pp. 62–67, doi : 10.1038 / nature13011 , PMID 24476820 , PMC 4106473 (free full text).
- ^ RA Forties, R. Bundschuh, MG Poirier: The flexibility of locally melted DNA. In: Nucleic acids research. Volume 37, number 14, August 2009, pp. 4580-4586, doi : 10.1093 / nar / gkp442 , PMID 19487242 , PMC 2724272 (free full text).
- ↑ R. Sapranauskas, G. Gasiunas, C. Fremaux, R. Barrangou, P. Horvath, V. Siksnys: The Streptococcus thermophilus CRISPR / Cas system provides immunity in Escherichia coli. In: Nucleic acids research. Volume 39, number 21, November 2011, pp. 9275-9282, doi : 10.1093 / nar / gkr606 , PMID 21813460 , PMC 3241640 (free full text).
- ^ S. Silas, P. Lucas-Elio, SA Jackson, A. Aroca-Crevillén, LL Hansen, PC Fineran, AZ Fire, A. Sánchez-Amat: Type III CRISPR-Cas systems can provide redundancy to counteract viral escape from type I systems. In: eLife. Volume 6, 08 2017, pp., Doi : 10.7554 / eLife.27601 , PMID 28826484 , PMC 5576922 (free full text).
- ↑ a b Type Ⅲ CRISPR-Cas Systems. In: Sino Biological. Retrieved February 14, 2019 .
- ↑ Rafael Molina, Stefano Stella, Mingxia Feng, Nicholas Sofos, Vykintas Jauniskis, Irina Pozdnyakova, Blanca López-Méndez, Qunxin She, Guillermo Montoya: Structure of Csx1-cOA4 complex reveals the basis of RNA decay in Type III-B CRISPR-Cas . In: Nature Communications. 10, 2019, doi : 10.1038 / s41467-019-12244-z .
- ↑ EV Kooni, YI Wolf: Is evolution Darwinian or / and Lamarckian? . Biology Direct 4:42 (2009)
- ^ R. Sorek, V. Kunin, P. Hugenholtz: CRISPR - a widespread system that provides acquired resistance against phages in bacteria and archaea . Nat Rev Microbiol 6: 181 (2007).
- ↑ H. Wang, H. Yang, CS Shivalila, MM Dawlaty, AW Cheng, F. Zhang, R. Jaenisch One-Step Generation of Mice Carrying Mutations in Multiple Genes by CRISPR / Cas-Mediated Genome Engineering , Cell, Volume 153, Issue 4, 9 May 2013, Pages 910-918.