Dihydrolevoglucosenone
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Dihydrolevoglucosenone | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 8 O 3 | |||||||||||||||
| Brief description |
clear colorless to light yellow liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 128.13 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density | ||||||||||||||||
| Melting point |
<−19.99 ° C at 1.013 hPa |
|||||||||||||||
| boiling point |
|
|||||||||||||||
| Vapor pressure |
0.28 hPa at 25 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Dihydrolevoglucosenone is a bicyclic , chiral , seven-membered heterocyclic cycloalkanone ( oxepanone ) which, as a bio-based and fully biodegradable aprotic-dipolar solvent , is a “green” alternative to problematic organic solvents in many applications, such as B. dimethylformamide (DMF), N-methyl-2-pyrrolidone (NMP) or sulfolane .
presentation
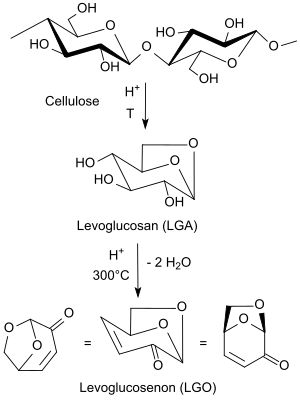
From lignin cellulose , lignocellulose or cellulose-inferior biomass such as wood waste or sawdust, the cellulose fraction can be prepared by acid-catalysed pyrolysis at 300 ° C via the intermediate levoglucosan (LGA) in the unsaturated anhydrosugar levoglucosenone (LGO) as a precursor of Dihydrolevoglucosenon (H 2 -LGO) be split. The yields of LGO are slightly over 10%; in addition, considerable amounts of charred or tar-like residues arise that can be used as fuel.
When cellulose is heated in tetrahydrofuran to 210 ° C. in the presence of low concentrations of sulfuric acid in an autoclave, up to 51% levoglucosenone is obtained in a so-called solvent-assisted pyrolysis. Levoglucosenone yields of up to 95% can be achieved under optimized conditions in laboratory batches.
Cellulose-containing waste from biorefineries already provides 6 to 8% LGO under microwave irradiation at 180 ° C for five minutes in addition to the usual decomposition products, such as. B. Hydroxymethylfurfural HMF, formic acid , formaldehyde , CO 2 and water.
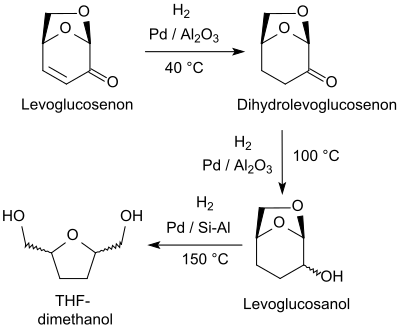
Hydrogenation of the α, β-unsaturated ketone levoglucosenone (LGO) over platinum metal catalysts, such as. B. Palladium on aluminum oxide Pd / Al 2 O 3 , at 40 ° C selectively leads to dihydrolevoglucosenone H 2 -LGO.
At higher temperatures, the saturated ketone H 2 -LGO is hydrogenated to the secondary alcohol levoglucosanol and further to tetrahydrofuran-2,5-dimethanol.
properties
Dihydrolevoglucosenone is a clear, colorless to pale yellow liquid with a comparatively high dynamic viscosity of 14.5 cP (for comparison DMF: 0.92 cP at 20 ° C, NMP: 1.67 cP at 25 ° C) and a mild smoky ketone-like odor, the is miscible with water and many organic solvents. The compound is stable at temperatures up to 140 ° C and against weak acids and bases. H 2 -LGO reacts with inorganic bases with aldol condensation . Dihydrolevoglucosenone is easily biodegradable (99% within 14 days), but reacts violently to oxidizing agents such as aqueous 30% hydrogen peroxide solution even at room temperature. The high boiling point of 227 ° C. is disadvantageous for the separation and work-up.
Applications
Dihydroglucosenone as a precursor
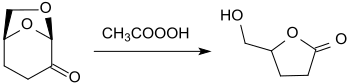
Dihydrolevoglucosenone is the starting compound for a number of secondary products that are of interest as bio-based building blocks for active ingredients or monomers for polycondensates . In the oxidation of H 2 -LGO with peracids , such as. B. peracetic acid in acetic acid is produced virtually quantitatively optically pure 5-Hydroxymethyldihydrofuranon [(S) - (+) - 4-hydroxymethyl-γ-butyrolactone], from which the earlier than HIV employed -Medikament zalcitabine (2'-3'-dideoxycytidine, ddC) is accessible.
In a two-stage hydrogenation process over platinum catalysts, initially at 60 ° C. and then at 180 ° C., mainly 1,6-hexanediol is obtained via several intermediate stages , which is used as a diol component in polyesters and polyurethanes or as a starting material for the diamine 1,6-diaminohexane .
With specific temperature control and the use of suitable palladium catalysts, tetrahydrofuran-2,5-dimethanol (THF-dimethanol) can be selectively obtained by hydrogenolysis of dihydroglucosenone via levoglucosanol , which is suitable as a biodegradable solvent and a bio-based precursor for 1,6-hexanediol (and 1, 6-diaminohexane).
Dihydroglucosenone as a novel polar solvent
Conventional, i.e. H. Aprotic dipolar solvents produced from fossil raw materials, such as dimethylformamide , dimethylacetamide , N-methyl-2-pyrrolidone (NMP), dichloromethane , acetonitrile , dimethyl sulfoxide and the like. a. are under increasing criticism because of their environmental profile (poor biodegradability, formation of NO x or SO x during combustion), their acute and chronic toxicity and their proven or suspected mutagenicity . The search for alternative “green” solvents from non-usable biomass or inexpensive renewable raw materials, which are accessible in high yield through highly efficient processes and which meet the performance profile of conventional solvents as far as possible, has triggered intensive research activities in industry and science worldwide.
A promising candidate as a “green” aprotic dipolar solvent could be dihydrolevoglucosenone. In several standard reactions in organic chemistry, e.g. B. the Menschutkin reaction , the Sonogashira coupling , the Suzuki-Miyaura coupling or the synthesis of organic ureas showed Dihydrolevoglucosenon comparable and z. T. better properties than the comparison solvents.
According to a statement by the management of the Australian Circa Group, dihydrolevoglucosenone performed worse in 35% of the tests carried out since 2015, comparable in 45% and better in 20% than NMP and similar solvents. The company produces small quantities of dihydrolevoglucosenone in a pilot plant set up together with the Norwegian company Norske Skog with a capacity of 50 metric tons per year (FC5) under the Cyrene TM brand in Tasmania . In a further step, commercial production of 5000 tons of Cyrene TM per year based on sawdust from the Tasmanian Boyer Mil is planned.
literature
- DS van Es: Study into alternative (biobased) polar aprotic solvents . Wageningen University, Wageningen 2017 ( wur.nl [PDF]).
- JH Clark, A. Hunt, C. Topi, G. Paggiola, J. Sherwood: Sustainable Solvents: Perspectives from Research, Business and Institutional Policy . Royal Society of Chemistry, London 2017, ISBN 978-1-78262-335-9 .
Individual evidence
- ↑ a b c d e f g h data sheet Cyrene, 99% from Sigma-Aldrich , accessed on January 5, 2018 ( PDF ).
- ↑ a b c d Circa: Data Sheet: Cyrene TM
- ↑ a b Patent US5112994 : Method of producing (S) -4-hydroxymethyl-γ-lactone. Filed September 17, 1990 , published May 12, 1992 , Applicants: Japan Tobacco Inc., Yuki Gosei Kogyo Co., Ltd., Inventors: K. Koseki, T. Ebata, H. Kawakami, H. Matsushita, K. Itoh, Y. Naoi.
- ↑ a b c James Sherwood, Mario de Bruyn, Andri Constantinou, Laurianne Moity, C. Rob McElroy, Thomas J. Farmer, Tony Duncan, Warwick Raverty, Andrew J. Hunt, James H. Clark: Dihydrolevoglucosenone (Cyrene) as a bio -based alternative for dipolar aprotic solvents . In: Chem. Commun. tape 50 , 2014, p. 9650-9652 , doi : 10.1039 / C4CC04133J .
- ↑ WS Trehanovsky, C. Wang, JM Ochaoda, C. Chang: A convenient procedure for the preparation of levoglucosenone from cellulose and the conversion of levoglucosenone to novel chiral derivatives . In: ACS Symposium Series . tape 841 , 2003, chap. 2 , p. 228-230 , doi : 10.1021 / bk-2003-0841.ch002 .
- ↑ F. Cao, TJ Schwartz, DJ McClelland, SH Krishna, JA Dumesic, GW Huber: Dehydration of cellulose to levoglucosenone using polar aprotic solvents . In: Energy Environ. Sci. tape 8 , 2015, p. 1808-1815 , doi : 10.1039 / C5EE00353A .
- ↑ Patent US9376451B1 : Method for selectively preparing levoglucosenone (LGO) and other anhydrosugars from biomass in polar aprotic solvents. Filed December 31, 2014 , published June 28, 2016 , applicant: Wisconsin Alumni Research Foundation, inventors: GW Huber, F. Cao, JA Dumesic, TJ Schwartz.
- ↑ M. De bruyn, J. Fan, VL Budarin, DJ Macquarrie, LD Gomez, R. Simister, TJ Farmer, WD Raverty, SJ McQueen-Mason, JH Clark: A new perspective in bio-refining: levoglucosenone and cleaner lignin from waste biorefinery hydrolysis lignin by selective conversion of residual saccharides . In: Energy Environ. Sci. tape 9 , 2016, p. 2571-2574 , doi : 10.1039 / c6ee01352j .
- ↑ a b c S.H. Krishna, DJ McClelland, QA Rashke, JA Dumesic, GW Huber: Hydrogenation of levoglucosenone to renewable chemicals . In: Green Chem. Band 19 , 2017, p. 1278-1285 , doi : 10.1039 / C6GC03028A .
- ↑ HJ Salavagione, J. Sherwood, M. De bruyn, VL Budarin, GJ Ellis, JH Clark, PS Shuttleworth: Identification of high performance solvents for the sustainable processing of graphene . In: Green Chem. Band 19 , 2017, p. 2550-2560 , doi : 10.1039 / C7GC00112F .
- ↑ M. Okabe, RC Sun, SYK Tam, LJ Todaro, DL Coffen: Synthesis of the dideoxynucleosides “ddC” and “CNT” from glutamic acid, ribonolactone, and pyrimidine bases . In: J. Org. Chem. Band 53 , no. 20 , 1988, pp. 4780-4786 , doi : 10.1021 / jo00255a021 .
- ↑ Patent US8889912B2 : Process for preparing 1,6-hexanediol. Registered on April 25, 2013 , published on November 18, 2014 , applicant: EI du Pont de Nemours and Company, inventors: AM Allgeier, DR Corbin, WIN De Silva, E. Korovessi, CA Menning, JC Ritter, SK Sengupta.
- ↑ Jiayue He, Kefeng Huang, Kevin J. Barnett, Siddarth H. Krishna, David M. Alonso, Zachary J. Brentzel, Samuel P. Burt, Theodore Walker, Williams F. Banholzer, Christos T. Maravelias, Ive Hermans, James A. Dumesic, George W. Huber: New catalytic strategies for α, ω- diols production from lignocellulosic biomass . In: Faraday Disc. Band 202 , 2017, p. 247-267 , doi : 10.1039 / C7FD00036G .
- ↑ FP Byrne et al .: Tools and techniques for solvent selection: Green solvent selection guides . In: Sustain. Chem. Proc. tape 4 , no. 7 , 2016, p. 1-24 , doi : 10.1186 / s40508-016-0051-z .
- ^ KL Wilson, AR Kennedy, J. Murray, B. Greatrex, C. Jamieson, AJB Wilson: Scope and limitations of a DMF bio-alternative within Sonogashira cross-coupling and Cacchi-type annulation . In: Beilstein J. Org. Chem. Volume 12 , 2016, p. 2005–2011 , doi : 10.3762 / bjoc.12.187 .
- ^ KL Wilson, J. Murray, C. Jamieson, AJB Watson: Cyrene as a bio-based solvent for the Suzuki-Miyaura cross-coupling . In: Synlett . tape 28 , 2017, p. A-E , doi : 10.1055 / s-0036-1589143 .
- ↑ L. Mistry, K. Mapesa, TW Bonsfield, JE Camp: Synthesis of ureas in the bio-alternative solvent Cyrene . In: Green Chem. Band 19 , 2017, p. 2123-2128 , doi : 10.1039 / C7GC00908A .
- ^ The future of sustainability at Circa. Specialty Chemicals Magazine, April 17, 2017, archived from the original ; accessed on January 5, 2018 .
- ↑ Press Release: Circa Group's Commercial Demonstration Plant Comes Online and Produces First Batch of 99% Pure Cyrene®. Press release at Sustainable Consult, February 12, 2019; accessed on March 24, 2019.
- ^ Government backs bio-based solvent project. Article on print21.com.au, March 1, 2018; accessed on March 24, 2019.