Tetrahydrofuran-2,5-dimethanol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| Simplified structural formula cis form and trans forms | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Tetrahydrofuran-2,5-dimethanol | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 12 O 3 | |||||||||||||||
| Brief description |
clear colorless oily liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 132.16 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density | ||||||||||||||||
| Melting point |
<−50 ° C |
|||||||||||||||
| boiling point |
|
|||||||||||||||
| Vapor pressure |
30 Pa (105 ° C) |
|||||||||||||||
| solubility |
completely miscible with water, ethanol , acetone , benzene , pyridine , DMSO and acetonitrile |
|||||||||||||||
| Refractive index |
1.4766 (25 ° C, 589 nm) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Tetrahydrofuran-2,5-dimethanol is a heterocyclic diol in which the five-membered oxolane ring has a hydroxymethyl group in the vicinity of the oxygen atom (in the 2- and 5-positions) .
The catalytic hydrogenation of hydroxymethylfurfural , an important platform chemical made from renewable raw materials, under suitable conditions predominantly delivers the cis -isomer ( meso- bis (hydroxymethyl) tetrahydrofuran).
THF-diol is used as a bio-based raw material for fuels , PVC - plasticizers (engl., Or as a molecular building block building block ) "green" for active substances, as well as monomers such. B. 1,6-hexanediol , hexamethylenediamine or caprolactam increasing interest.
presentation
The synthesis of 2,5-bis (hydroxymethyl) tetrahydrofuran by hydrogenation of hydroxymethylfurfural in the presence of Raney nickel in "excellent yields" was described as early as 1945 and the product was identified as the cis isomer (= meso form).
Because it is carried out in diethyl ether at 160 ° C and 14 MPa pressure for 20 hours, the process is not suitable for larger batches. The search for milder reaction conditions with high selectivity for THF-diol is described in several patents and publications. In the meantime, in the hydrogenation with nickel / palladium catalysts at 40 ° C. and 8 MPa hydrogen pressure, yields of 96% have been achieved after 2 hours. With longer reaction times , higher temperatures and acidic pH values, cleavage of the furan ring and the formation of C 6 polyols must be expected. The cis - trans ratio found is 90:10 to 98: 2.
Continuous one- or two-stage processes for the preparation of tetrahydrofuran-2,5-dimethanol are also discussed. Attention is drawn to the only partially reversible deactivation of the nickel catalyst.
Under gentler conditions, e.g. B. at 35 ° C and 0.8 MPa hydrogen pressure on a platinum contact in an aqueous medium, only the aldehyde group of hydroxymethylfurfural is hydrogenated and 2,5-bis (hydroxymethyl) furan is obtained practically quantitatively.
The production of THF-diol by hydrogenation of tetrahydrofuran-2,5-dicarboxylic acid dimethyl ester (by esterification of 2,5-furandicarboxylic acid with methanol and subsequent hydrogenation in the presence of Raney nickel) on a copper chromite contact or with lithium aluminum hydride is also described, but unproductive.
properties
Tetrahydrofuran-2,5-dimethanol is a clear, oily, colorless to pale yellow liquid that is completely miscible with water and many organic solvents. The compound is hygroscopic and liquid in a temperature range of over 300 ° C.
Applications
Tetrahydrofuran-2,5-dimethanol as solvent
THF-diol is an aprotic dipolar solvent and can replace ethylene glycol and propylene glycol in latex paints . Emissions of volatile organic compounds (VOC) are significantly reduced.
Tetrahydrofuran-2,5-dimethanol as a starting material for functional molecules
The synthesis of pharmacologically active heterocyclic tropane analogs from THF diol via the di-tosylate , which was first described by Arthur C. Cope , confirmed the almost exclusively cis configuration of the diol.
The two hydroxymethyl groups of the THF-diol can be converted into disulfonates and these can be converted into dinitriles with sodium cyanide . From the dinitrile tetrahydrofuran-2,5-diacetonitrile are by partial hydrogenation, for. B. with DIBAL the dialdehyde, the dicarboxylic acid by hydrolysis and the diamine by complete hydrogenation .
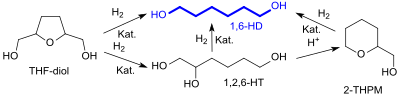
Hydrogenation and hydrogenolysis reactions on tetrahydrofuran-2,5-dimethanol, which produce different products depending on the reaction conditions, have recently been intensively worked on.
In addition to the direct route, the hydrogenation also leads to 1,6-hexanediol (1,6-HD) via 1,2,6-hexanediol (1,2,6-HT) and 2-tetrahydropyranmethanol (2-THPM).
Tetrahydrofuran-2,5-dimethanol as a monomer for polyester
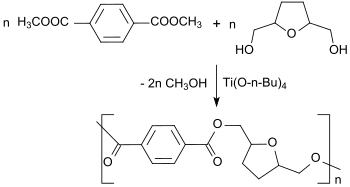
The transesterification of 2,5-bis (hydroxymethyl) tetrahydrofuran with dimethyl terephthalate in the presence of the transesterification catalyst titanium tetrabutanolate produces polyesters in a polycondensation reaction .
The polyesters obtained are amorphous or semi-crystalline and can be processed thermoplastically, so that they have interesting similarities to polyethylene terephthalate .
THF-diol as a molecular building block from renewable resources has received some primarily scientific attention, but has not yet found industrial application. The most promising future use as a bio-based raw material source for 1,6-hexanediol, a possible precursor for the polyamide monomers 1,6-diaminohexane (for polyamide 6.6) and caprolactam (for polyamide 6 ) , currently appears to be the most promising .
As a result of the future large-volume and then inexpensive availability of the platform chemical hydroxymethylfurfural (HMF), its hydrogenation product tetrahydrofuran-2,5-dimethanol could become significantly more important as a key compound for bio-based monomers for polyesters, polyamides and possibly also for polyurethanes .
literature
- F. Cavani, S. Albonetti, F. Basile, A. Gandini (Eds.): Chemicals and Fuels from Bio-Based Building Blocks, Volume 1 . Wiley-VCH, Weinheim 2016, ISBN 978-3-527-33897-9 .
- I. Delidovich, PJC Hausoul, L. Deng, R. Pfützenreuther, M. Rose, R. Palkovits: Alternative monomers based on lignocellulose and their use of polymer production . In: Chem. Rev. Band 116 , no. 3 , 2016, p. 1540-1599 , doi : 10.1021 / acs.chemrev.5b00354 .
- Z. Fang, RL Smith, Jr., X. Qi (Eds.): Production of Platform Chemicals from Sustainable Resources . Springer Nature Singapore Pte. Ltd., Singapore 2017, ISBN 978-981-10-4171-6 .
- IT Horváth, M. Malacria (Ed.): Advanced Green Chemistry, Part 1: Greener Organic Reactions and Processes . World Scientific Publishing Co. Pte. Ltd., Singapore 2018, ISBN 978-981-3228-10-8 .
Individual evidence
- ↑ a b c Patent WO2016102361A1 : Polyesters from aromatic carboxylic diacid and 2,5-bis (hydromethyl) tetrahydrofuran. Registered on December 18, 2015 , published on June 30, 2016 , applicant: Rhodia Operations, inventor: S. Jeol.
- ↑ a b c d William M. Haynes: CRC Handbook of Chemistry and Physics, 97th Edition . CRC Press, Boca Raton, FL, USA 2015, ISBN 978-1-4987-5428-6 , pp. 3-26 .
- ↑ a b Entry on 2,5-bis (hydroxymethyl) tetrahydrofuran in the GESTIS substance database of the IFA , accessed on January 22, 2018 (JavaScript required)
- ↑ a b c d e f A.C. Cope, WN Baxter: Aminoalcohols Containing the 8-Oxa-3-azabicyclo [3.2.1] octane Ring System and Their Benzoates . In: J. Am. Chem. Soc. tape 77 , no. 2 , 1955, pp. 393–396 , doi : 10.1021 / ja01607a049 .
- ↑ a b Patent WO2013188252A2 : Diallyl ethers of 2,5-bis (hydroxymethyl) tetrahydrofuran and processes for making the same. Filed June 12, 2013 , published December 19, 2013 , applicant: Archer Daniels Midland Co., inventor: K. Stensrud.
- ↑ a b c T.J. Connolly et al .: Efficient synthesis of 8-oxa-3-aza-bicyclo [3.2.1] octane hydrochloride . In: Org. Process Res. Dev. Band 14 , 2010, p. 459-465 , doi : 10.1021 / op9002642 .
- ↑ Entry on tetrahydrofuran-2,5-diyldimethanol in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on January 28, 2018. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Patent WO2009141166A1 : composition Fuel. Applied on May 19, 2008 , published on November 26, 2009 , Applicant: Furanix Technologies BV, Inventor: GJM Gruter, E. De Jong.
- ↑ Patent WO2015032794A1 : Tetrahydrofuran derivatives and their use as plasticizers. Registered on September 4, 2013 , published on March 12, 2015 , applicant: BASF SE, inventor: J. Wagner, B. Breitscheidel, MA Bohn, B. Blank, A. Kindler.
- ↑ a b c T. Buntara, S. Noel, PH Phua, J. Melián-Cabrera, JG de Vries, HJ Heeres: Caprolactam from renewable resources: Catalytic conversion of 5-hydroxymethylfurfural into caprolactone . In: Angew. Chem. Int. Ed. tape 50 , no. 31 , 2011, p. 7083–7087 , doi : 10.1002 / anie.201102156 .
- ^ WN Haworth, WGM Jones, LF Wiggins: 1. The conversion of sucrose into furan compounds. Part II. Some 2: 5-disubstituted tetrahydrofurans and their products of ring scission . In: J. Chem. Soc. tape 0 , 1945, p. 1-4 , doi : 10.1039 / JR9450000001 .
- ↑ Patent US3040062 : Process for preparing 2,5-bis hydroxymethyl tetrahydrofuran. Applied on November 14, 1960 , published June 19, 1962 , applicant: Atlas Chemical Industries, Inc., inventor: RA Hales.
- ↑ Y. Nakagawa, K. Tomishige: Total hydrogenation of furan derivatives over silica-supported Ni-Pd alloy Catalyst . In: Catal. Commun. tape 12 , 2010, p. 154–156 , doi : 10.1016 / j.catcom.2010.09.003 .
- ^ R. Alamillo, M. Tucker, M. Chia, Y. Pagán-Torres, J. Dumesic: The selective hydrogenation of biomass-derived 5-hydroxymethylfurfural using heterogeneous catalysts . In: Green Chem. Band 14 , no. 5 , 2010, p. 1413-1419 , doi : 10.1039 / C2GC35039D .
- ^ S. Lima, D. Chadwick, K. Hellgardt: Towards sustainable hydrogenation of 5- (hydroxymethyl) furfural: a two-stage continuous process in aqueous media over RANEY R catalysts . In: RSC Advances . tape 7 , 2017, p. 31401-31407 , doi : 10.1039 / C7RA03318A .
- ↑ M. Chatterjee, T. Ishizaka, H. Kawanami: Selective hydrogenation of 5-hydroxymethylfurfural to 2,5-bis- (hydroxymethyl) furan using Pt / MCM-41 in an aqueous medium: a simple approach . In: Green Chem. Band 16 , no. 11 , 2014, p. 4734-4739 , doi : 10.1039 / C4GC01127A .
- ↑ Patent US7579490B2 : Conversion of 2,5- (hydroxymethyl) furaldehyde to industrial derivatives, purification of the derivatives, and industrial uses therefor. Filed November 15, 2005 , published August 25, 2009 , applicant: Archer-Daniels-Midland Company, inventor: AJ Sanborn, PD Bloom.
- ↑ Patent US20160207894A1 : Synthesis of diacids, dialdehydes, or diamines from THF-diols. Filed September 25, 2014 , published July 21, 2016 , applicant: Archer Daniels Midland Company, inventor: K. Stensrud.