Iron (II) sulfate
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
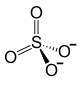
|
||||||||||
| General | ||||||||||
| Surname | Iron (II) sulfate | |||||||||
| other names |
|
|||||||||
| Molecular formula | FeSO 4 | |||||||||
| Brief description |
|
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| Drug information | ||||||||||
| ATC code | ||||||||||
| properties | ||||||||||
| Molar mass |
|
|||||||||
| Physical state |
firmly |
|||||||||
| density |
|
|||||||||
| Melting point |
Decomposition: above 400 ° C |
|||||||||
| solubility |
easily in water (256 g l −1 at 20 ° C, anhydrous) |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| Toxicological data | ||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Iron (II) sulfate (also ferrous sulfate , green salt , iron vitriol , formerly also Green Galitzenstein ) is a divalent iron salt of sulfuric acid . The name green salt for iron (II) sulfate heptahydrate (FeSO 4 · 7 H 2 O) is derived from the greenish color of the salt containing water of crystallization .
Occurrence and manufacture
It is made by heating powdered iron in 20% sulfuric acid :
After the evolution of hydrogen has ended, the mixture is concentrated and filtered hot.
Iron (II) sulfate can also be obtained commercially by oxidizing pyrite.
In the production of titanium dioxide with the sulphate process , iron (II) sulphate is also produced in large quantities as a secondary product . The largest quantities arise in Germany in the production plants in Leverkusen and Nordenham of Kronos International, Inc. It is also produced when pickling iron sheets .
Iron sulphate occurs naturally as the mineral melanterite ; it is a weathering product of pyrite . It occurs in different hydrate forms, which also occur in the wild.
- FeSO 4 H 2 O (mineral: Szomolnokite , relatively rare)
- FeSO 4 4 H 2 O (mineral: rozenite , white, relatively common, dehydration product of melanterite )
- FeSO 4 5 H 2 O (mineral: siderotile , relatively rare)
- FeSO 4 6 H 2 O (mineral: ferrohexahydrite , relatively rare)
- FeSO 4 7 H 2 O (mineral: melanterite , blue, relatively common)
properties
Iron (II) sulfate crystallizes from aqueous solutions as light green crystals that contain 7 mol of water of crystallization : FeSO 4 · 7 H 2 O, hence the name iron (II) sulfate heptahydrate. It is light blue only in the pure state without crystal water (FeSO 4 ). The greenish color is caused by partial oxidation to Fe 3+ . As the temperature rises, water of crystallization is gradually released. The heptahydrate begins to split off water of crystallization at 60 ° C, and then that of the hydrate from 300 ° C. The crystals weather in dry air, this elimination of water of crystallization succeeds completely with prolonged heating above 70 ° C, the monohydrate is formed as a colorless powder FeSO 4 · H 2 O, which with strong heating from about 400 ° C to iron (III) oxide , Sulfur dioxide and sulfur trioxide break down. Iron sulfate is readily soluble in water, but practically insoluble in ethanol and acetone . The aqueous solution of iron (II) sulfate is acidic. The solution, however, is unstable, with some basic iron (III) sulfate (iron (III) hydroxysulfate) being formed through oxidation with atmospheric oxygen .
Historical use
Copper water
Kopper water , Kopper Water , copper water are old names for impure (copper-containing) vitriol. In the Middle Ages, copper water and iron vitriol were sometimes equated in the commercial sector ; the salts served as tanning and pickling agents , along with alums and other copper, iron, zinc, chromium and tin salts .
Copper water was obtained in copper mining and shipped in barrels through Hanseatic trade. In 1501, according to the text "Summarized extract and description of the Khauf trade and Schefleuth in Lands Bayrn", copper water belonged to the goods that were cleared on the Loisach.
Iron gall ink
Persians , Medes , Assyrians and Hebrews wrote on untanned hides with ink made from soot and oil, which was easily rubbed off or washed off; since about the 3rd century BC was Eisengallustinte , a sulfate of iron (II), galls, water and gum arabic prepared ink , as indelible black ink for writing on parchment and later paper in use.
Remedies
Iron sulphate is found in the formulation of well-known remedies from the Middle Ages, such as Theriaks , which consisted of over 60 ingredients (herbal extracts, opium, spices, snake meat , etc.).
safety instructions
Iron (II) sulfate has an acutely irritating to corrosive effect on skin and mucous membranes. The sulfate can be absorbed through skin contact as well as orally and through the airways . Oral ingestion of relevant amounts above about 20 mg / kg body weight can cause acute damage to the gastrointestinal tract , the liver and the cardiovascular system . Acidoses with fatal consequences are possible at high doses of 180–300 mg / kg . In the case of chronic exposure , accumulation with tissue damage to internal organs can occur.
In children, oral intake of 390 mg / kg (1964) resulted in loss of appetite and sleepiness with fatal consequences ( LD Lo ); In a poisoning case from 1982 an oral LD Lo of 699 mg / kg was determined. The oral LD 50 values for animals are 319 mg / kg (rat), 600 mg / kg (dog) and 680 mg iron sulfate per kg body weight (mouse).
From 400 ° C iron (II) sulphate decomposes with the formation of sometimes toxic products such as sulfur dioxide and sulfur trioxide .
Modern use
- In the laboratory and in synthetic chemistry , it is an important starting material for the production of other iron compounds .
- For desulfurization :
- In the case of long sewer lines and sewer pressure pipes, there are always odor problems in the warmer seasons. The cause is the formation of hydrogen sulfide . The formation of hydrogen sulfide can be prevented with iron (II) sulfate.
- Biogas plants also have to struggle with the formation of hydrogen sulfide. Here iron (II) sulfate is used for biogas desulphurisation .
- It is also used for coal desulfurization.
- In analytical chemistry, nitrates and nitrites are qualitatively detected with iron sulfate solution, namely by means of the so-called ring test , when the iron sulfate-containing sample solution is layered under with concentrated sulfuric acid, a brown ring of iron nitrososulfate forms at the interface. The quantitative titration with iron sulphate standard solution is rarely used because of the instability of the solution with regard to oxidation by atmospheric oxygen.
- As a neutralizer :
- It serves as a precipitant and flocculant in wastewater treatment . Large sewage treatment plants in particular often use iron (II) sulphate to remove phosphate . This is because the preparation of a meterable iron (II) sulfate solution requires a complex dissolving bunker . Smaller wastewater treatment plants usually use industrially produced bivalent or trivalent solutions that can be dosed from a storage tank. For the production of the trivalent iron (III) chloride sulfate solution, iron (II) sulfate is used as the raw material.
- In the construction industry it is added to chromate-containing cement to reduce Cr VI to Cr III as a chromate reducer .
- To protect wood from moss and fungal growth outdoors.
- As a source of iron and sulfur :
- In the fertilizer sector, dried iron (II) sulfate is added to the lawn fertilizer . This leads to the control of mosses and nudibranchs .
- Can also be used to protect wood from moss and fungus formation outdoors (with graying of the wood as a side effect).
- To combat chlorosis in viticulture.
- As a colorant :
- In the past, a gall apple brew (which contains gallic acid ) was used to make black iron gall ink with the addition of iron sulfate . In the long run, however, this will cause the parchment to become damaged by ink , as the iron sulfate releases sulfuric acid when it is weathered .
- As fertilizer :
- In agriculture.
- As a medicinal substance :
- Nowadays, iron (II) sulfate is used as a highly effective blood-forming agent in the form of film-coated tablets.
Web links
Individual evidence
- ↑ Entry on FERROUS SULFATE in the CosIng database of the EU Commission, accessed on March 21, 2020.
- ↑ a b c d e f g h i j k l Entry on iron (II) sulfate in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ Entry on Iron sulphate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ^ The Journal of Pediatrics . Vol. 69, Pg. 663, 1966.
- ↑ a b c d e Entry on iron (II) sulfate in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ↑ Data sheet iron (II) sulfate (PDF) from Carl Roth , accessed on December 14, 2010.
- ^ British Journal of Pharmacology and Chemotherapy . Vol. 24, Pg. 352, 1965.
- ↑ a b Journal of Pediatrics. Vol. 64, Pg. 218, 1964.
- ^ AF Holleman , E. Wiberg : Textbook of Inorganic Chemistry . 57-70 Edition. Walter de Gruyter, Berlin 1964, p. 550.
- ↑ Brockhaus' Konversationslexikon: Kupferwasser - Cupola furnace
- ↑ Sabine Struckmeier: Textile dyeing from the late Middle Ages to the early modern period (14th-16th centuries): A scientific-technical analysis of German-language sources . Waxmann Verlag, 2011, ISBN 978-3-8309-7527-4 , p. 73 ( limited preview in Google Book search).
- ↑ Rafting company Josef Seitner: Raft trip on Loisach and Isar , accessed on June 5, 2013.
- ^ Tilman Nagel, Lower Saxony State and University Library Göttingen (ed.): Encounters with Arabia .: 250 years of Arabic studies in Göttingen . Wallstein Verlag, Göttingen 1998, ISBN 3-89244-097-2 , p. 54 ( limited preview in Google Book search).
- ^ Journal of Forensic Sciences. Vol. 27, Pg. 955, 1982.
- ↑ Felix R. Althaus: Textbook of pharmacology and toxicology for veterinary medicine. Georg Thieme Verlag, 2007, ISBN 978-3-8304-1070-6 , p. 215 ( limited preview in the Google book search).
- ↑ Pharmacy info leaflet iron sulfate Lomapharm 100mg In: apotheken-umschau.de .
- ↑ ÖKO-TEST yearbook toddlers for 2008, test iron preparations ( memento of the original dated November 24, 2016 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. In: oekotest.de .




