Radical (chemistry)
As radicals are called atoms or molecules with at least one unpaired valence electron , which are usually very reactive. Radicals are represented with a “point”, for example nitrogen monoxide (NO • ), which symbolizes the free electron. If a radical contains several unpaired electrons, one speaks of diradical (also biradical ), triradical , etc. Radicals play an important role in certain oxidation processes , in chain polymerizations and in some substitution reactions .
nomenclature
In the nomenclature radicals are named with the ending -yl, for biradicals -ylidene is used. Exceptions are the diradicals methylene , silylene and aminylene . Examples are -oxyl for compounds with free radical oxygen (e.g. 2,2,6,6-tetramethylpiperidinyloxyl ) or thiyl radicals with free radical sulfur atom.
Emergence
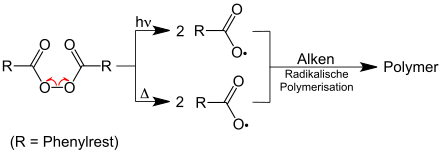
Radicals are formed by:
- Heat ( thermolytic bond cleavage)
- UV radiation , which causes the homolytic cleavage of a covalent bond ( photolysis )
- X-rays and other ionizing radiation
- Electrochemically through oxidation or reduction
To initiate radical reactions in chemical synthesis, so-called radical starters are often added to the reaction mixture. These are molecules that are particularly easy to split into radicals - for example by exposure to ultraviolet light. Examples of radical initiators are: azobis iso butyronitrile , dibenzoyl , dilauroyl , di- tert butyl peroxide , diisopropyl and potassium .
Reactivity
Reactive radicals
Since most radicals react exergonically , they are very reactive and therefore short-lived (<1 second). The unpaired electron is usually located on C , N , O and Hg atoms or halogens .
Unreactive radicals
Radicals are also known which do not react immediately and which sometimes even exist as isolable substances over a certain period of time. An example of these “stable radicals” is the triphenylmethyl radical. Like other unreactive radicals, it is in equilibrium with its dimer in solution. However, the dimer of the triphenylmethyl radical is not hexaphenylethane , as Gomberg assumed, but the 3-diphenylmethylene-6-triphenylmethyl-cyclohexa-1,4-diene shown in the figure. Factors that lead to stable radicals are, on the one hand, a resonance stabilization of the radical and, on the other hand, an obstruction of dimerization, for example by sterically demanding substituents. Stable radicals also occur in nature. For example, the enzyme ribonucleotide reductase contains a tyrosyl radical with a half-life of 4 days.
Carbon radicals
C-centered radicals show increasing stability in the series: primary C atom <secondary C atom <tertiary C atom, which is due to inductive effects and hyperconjugation . In addition, sp³-hybridized carbon radicals are more stable than radical centers in which the carbon exhibits sp² or sp hybridization. Also aryl - or allyl groups stabilize the radical.
Well-known examples
- Oxygen O 2 - this oxygen molecule contains two unpaired electrons (biradical • OO • ; the Lewis formula O = O does not fully and correctly reflect the bonding situation) and is represented as a paramagnetic triplet in the magnetic field. However, the reactivity of this biradical is limited, since the principle of the preservation of the spin in chemical reactions in most cases first requires an excitation to singlet oxygen.
- Nitric oxide • NO - a radical recognized as a messenger substance . Nitric oxide is a component of nitrous gases .
- Hydroxyl radical • OH - the most reactive and most important radical in the atmosphere (important for breaking down air pollutants )
- Chlorine radicals Cl • - arise through homolytic cleavage of the chlorine-chlorine bond from molecular chlorine (Cl 2 ) and are reactive intermediates in the photochlorination of alkanes and in the side chain chlorination ( SSS rule ) of alkyl-substituted aromatics. They are also released from chlorofluorocarbons when exposed to light and are involved in the destruction of the ozone layer .
- Bromine radicals Br • - are formed by homolytic cleavage of the bromine-bromine bond from molecular bromine (Br 2 ) and are reactive intermediates in the photobromination of alkanes and in the side chain bromination (SSS rule) of alkyl-substituted aromatics
- TEMPO - a stable organic radical that is used, among other things, as an oxidizing agent
Radical detection
Radicals can be detected directly or indirectly:
- If a molecule has unpaired electrons, the spin of the electrons in a magnetic field can adopt two different orientations. This can be demonstrated by ESR spectroscopy.
- Since most radicals are very reactive, they can be characterized by the product created with a radical scavenger:
- Spin-trap reagents such as tert -Bu-N = O can be used for very short-lived radicals . After the addition of the radical, the persistent nitroxide is formed, which can be examined spectroscopically using ESR.
- The dimerization of two radicals creates a stable molecule that can be isolated and characterized. Example: Two methyl radicals (• CH 3 ) dimerize to ethane (H 3 C – CH 3 ).
Radicals in Biology
Radicals, such as reactive oxygen species (ROS), play an important role in a large number of biological processes, but can also cause cell damage, which, among other things , can contribute to the development of cancer . The oxidation of various substances mediated by free radicals is also assigned an important role in the development of arteriosclerosis , Alzheimer's disease , liver damage from alcohol and emphysema from tobacco smoke . Among the intracellular signaling pathways that are activated by free radicals, the NF-κB signaling pathway is one of the most important.
Protection against the effects of radicals is essential, the body therefore has effective defense and repair mechanisms in the form of enzymes, hormones or other classes of substances that minimize the harmful effects. Antioxidants such as epigallocatechin gallate , superoxide dismutase , glutathione peroxidase , vitamin A , vitamin C , vitamin E , coenzyme Q10 , flavonoids such as taxifolin and anthocyanins are involved in these defense mechanisms . Also, bilirubin , and uric acid should be able to neutralize certain free radicals. The hormone melatonin is also considered a radical scavenger against oxidative stress . The strongest known antioxidant , the hydride ion H - , plays an important role in the citric acid cycle and in many redox reactions of the metabolism.
Radicals play a role in the so-called "wear and tear" theories of the aging process in the body, so that active substances against oxidative stress are being discussed as agents against aging (→ theory of free radicals ). It is known that the cells of birds are far better able to withstand free radicals. However, since only the influence of synthetic antioxidants was examined, no conclusions should be drawn about the possible effects of fruit and vegetables. In 2007, a JAMA editorial called for further randomized studies to establish the effects of vitamin C and selenium. A very large meta-analysis by the Cochrane Collaboration (2007–2012), which has been updated several times since then, finally found no positive effects of vitamin-containing food supplements; on the contrary, the mortality in the verum group rose slightly.
Historical meaning
When the theory prevailed at the beginning of the 19th century that all matter is made up of atoms (see John Dalton ), the term radical was used by eminent chemists such as Lavoisier and Wöhler to denote polyatomic molecules that are involved in chemical reactions such as Individual atoms behaved. Auguste Laurent used the term radical for the first time to denote atoms and groups of atoms in nuclear theory (chemistry) . For example, the cyanate ion , which is made up of three atoms, often behaves like a chloride ion . An ammonium ion, which consists of five atoms, often behaves like the ion of an alkali metal . That is why cyanate and ammonium ions, among others, were called radicals. See also: radical theory .
literature
- Christoph Rüchardt: Radicals. A chemical theory in a historical perspective. In: Meeting reports of the Heidelberg Academy of Sciences, mathematical and natural science class. 1992, pp. 319-345 ( full text ).
- Ivo E. Dreosti (Ed.): Trace Elements, Micronutrients, and Free Radicals. Humana Press, Totowa (New Jersey) 1991, ISBN 0-89603-188-8 .
Web links
- Acyclic hydrocarbons (mono-, di- and trivalent radicals) ( Memento from January 12, 2012 in the Internet Archive )
swell
- ↑ D. Hellwinkel: The systematic nomenclature of organic chemistry. An instruction manual . 4th edition. Springer, Berlin 1998, ISBN 978-3-540-63221-4 , p. 92.
- ^ MD Lechner, K. Gehrke, EH Nordmeier: Makromolekulare Chemie . 4th edition, Birkhäuser Verlag, ISBN 978-3-7643-8890-4 , p. 54.
- ^ Siegfried Hauptmann : Organic chemistry . 2nd edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig 1985, ISBN 3-342-00280-8 , p. 281.
- ↑ Ulrich Lüning: Organic reactions - An introduction to the reaction pathways and mechanisms . 2nd Edition. Spektrum, Munich 2007, ISBN 978-3-8274-1834-0 , pp. 21 .
- ↑ Yu. A. Vladimirov, EV Proskurnina, EM Demin, NS Matveeva, OB Lubitskiy, AA Novikov, D. Yu. Izmailov, AN Osipov, VP Tikhonov, VE Kagan: Dihydroquercetin (taxifolin) and other flavonoids as inhibitors of free radical formation at key stages of apoptosis . In: Biochemistry (Moscow) . tape 74 , no. 3 , 2009, p. 301-307 , doi : 10.1134 / S0006297909030092 , PMID 19364325 .
- ↑ Goran Bjelakovic, Dimitrinka Nikolova, Lise Lotte Gluud, Rosa G. Simonetti, Christian Gluud: Mortality in Randomized Trials of Antioxidant Supplements for Primary and Secondary Prevention . In: JAMA: The Journal of the American Medical Association . tape 297 , no. 8 , January 28, 2007, p. 842-857 , doi : 10.1001 / jama.297.8.842 .
- ↑ G. Bjelakovic, D. Nikolova, LL Gluud, RG Simonetti, C. Gluud: Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. In: Cochrane database of systematic reviews (online). Volume 3, 2012, ISSN 1469-493X , S. CD007176, doi: 10.1002 / 14651858.CD007176.pub2 , PMID 22419320 (review article).
- ^ John Buckingham: Chasing the molecule . Sutton, Stroud 2004, ISBN 0-7509-3345-3 .