Turanose: Difference between revisions
Content deleted Content added
No edit summary |
|||
| (32 intermediate revisions by 27 users not shown) | |||
| Line 1: | Line 1: | ||
{{chembox |
{{chembox |
||
| Verifiedfields = changed |
|||
|ImageFile= |
|||
| Watchedfields = changed |
|||
| ⚫ | |||
| verifiedrevid = 426915255 |
|||
|IUPACName= |
|||
| Name = {{sm|d}}-Turanose |
|||
| ⚫ | |||
| ImageFile = Turanose.png |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
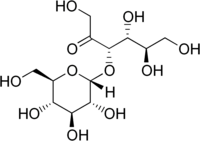
| IUPACName = α-<small>D</small>-glucopyranosyl-(1→3)-α-<small>D</small>-fructofuranose |
|||
| ⚫ | |||
| ⚫ | |||
| SMILES=C(C1C(C(C(C(O1)OC(C(C(CO)O)O)C(=O)CO)O)O)O)O |
|||
| Reference = <ref>[https://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=5460935 Turanose - Compound Summary], [[PubChem]]</ref> |
|||
| ⚫ | |||
| SystematicName = (3''S'',4''R'',5''R'')-1,4,5,6-tetrahydroxy-3-[(2''R'',3''R'',4''S'',5''S'',6''R'')-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyhexan-2-one |
|||
| ⚫ | |||
| CASNo_Ref = {{cascite|correct|??}} |
|||
| ⚫ | |||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
| UNII = 6D600ARY3R |
|||
| ⚫ | |||
| ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} |
|||
| ChemSpiderID = 4574343 |
|||
| SMILES = O=C([C@@H](O[C@H]1O[C@@H]([C@@H](O)[C@H](O)[C@H]1O)CO)[C@H](O)[C@H](O)CO)CO |
|||
| InChI = 1/C12H22O11/c13-1-4(16)7(18)11(5(17)2-14)23-12-10(21)9(20)8(19)6(3-15)22-12/h4,6-16,18-21H,1-3H2/t4-,6-,7-,8-,9+,10-,11-,12-/m1/s1 |
|||
| InChIKey = RULSWEULPANCDV-PIXUTMIVBJ |
|||
| StdInChI_Ref = {{stdinchicite|changed|chemspider}} |
|||
| StdInChI = 1S/C12H22O11/c13-1-4(16)7(18)11(5(17)2-14)23-12-10(21)9(20)8(19)6(3-15)22-12/h4,6-16,18-21H,1-3H2/t4-,6-,7-,8-,9+,10-,11-,12-/m1/s1 |
|||
| StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} |
|||
| StdInChIKey = RULSWEULPANCDV-PIXUTMIVSA-N |
|||
| ⚫ | |||
}} |
}} |
||
|Section2={{Chembox Properties |
| Section2 = {{Chembox Properties |
||
| |
| Formula=C<sub>12</sub>H<sub>22</sub>O<sub>11</sub> |
||
| |
| MolarMass=342.30 g/mol |
||
| |
| Appearance= |
||
| |
| Density= |
||
| |
| MeltingPt= |
||
| |
| BoilingPt= |
||
| |
| Solubility= |
||
}} |
}} |
||
|Section3={{Chembox Hazards |
| Section3 = {{Chembox Hazards |
||
| |
| MainHazards= |
||
| |
| FlashPt= |
||
| AutoignitionPt = |
|||
| Autoignition= |
|||
}} |
}} |
||
}} |
}} |
||
D-(+)-Turanose is a reducing disaccharide. It is an analog of sucrose not metabolized by higher plants, but rather acquired through the action of sucrose transporters for intracellular carbohydrate signaling. In addition to its involvement in signal transduction, D-(+)-Turanose can also be used as a carbon source by many organisms including numerous species of bacteria and fungi. <ref> |
|||
'''Turanose''' is a [[Redox|reducing]] [[disaccharide]]. The {{sm|d}}-isomer is naturally occurring. Its systematic name is α-{{sm|d}}-glucopyranosyl-(1→3)-α-{{sm|d}}-fructofuranose. It is an analog of [[sucrose]] not [[Metabolism|metabolized]] by [[Vascular plant|higher plants]], but rather acquired through the action of sucrose transporters for intracellular [[carbohydrate]] signaling. In addition to its involvement in signal transduction, {{sm|d}}-(+)-turanose can also be used as a carbon source by many organisms including numerous species of [[bacteria]] and [[fungus|fungi]].<ref>{{cite journal | author = Sinha, A.K.| title = Metabolizable and non-metabolizable sugars activate different signal transduction pathways in tomato | journal = Plant Physiol | volume = 128 | pages = 1480–1489 | year = 2002 | doi = 10.1104/pp.010771 | pmid = 11950996 | issue = 4 | pmc = 154275|display-authors=etal}}</ref><ref>{{cite journal | author = Gonzali, S.| title = A turanose-insensitive mutant suggests a role for WOX5 in auxin homeostasis in Arabidopsis thaliana | journal = Plant J | volume = 44 | pages = 633–645 | year = 2005 | doi = 10.1111/j.1365-313X.2005.02555.x | pmid = 16262712 | issue = 4|display-authors=etal| doi-access = free }}</ref><ref>{{cite journal | author = Sivitz, A.B.| title = Arabidopsis sucrose transporter AtSUC9. High-affinity transport activity, intragenic control of expression, and early flowering mutant phenotype | journal = Plant Physiol | volume = 143 | pages = 188–198 | year = 2007 | doi = 10.1104/pp.106.089003 | pmid = 17098854 | issue = 1 | pmc = 1761979|display-authors=etal}}</ref><ref>{{cite journal | author = Loreti, E.| title = Glucose and disaccharide-sensing mechanisms modulate the expression of α-amylase in barley embryos | journal = Plant Physiol | volume = 123 | pages = 939–948 | year = 2000 | doi = 10.1104/pp.123.3.939 | pmid = 10889242 | issue = 3 | pmc = 59056|display-authors=etal}}</ref><ref> [http://www.sigmaaldrich.com/catalog/search/ProductDetail/SIGMA/T2754 D-Turanose] at [[Sigma-Aldrich]]</ref> |
|||
Sinha, A.K., et al., Metabolizable and non-metabolizable sugars activate different signal transduction pathways in tomato. Plant Physiol. 128, 1480-1489, (2002)</ref><ref> |
|||
Gonzali, S., et al., A turanose-insensitive mutant suggests a role for WOX5 in auxin homeostasis in Arabidopsis thaliana. Plant J. 44, 633-645, (2005)</ref><ref> |
|||
Sivitz, A.B., et al., Arabidopsis sucrose transporter AtSUC9. High-affinity transport activity, intragenic control of expression, and early flowering mutant phenotype. Plant Physiol. 143, 188-198, (2007)</ref><ref> |
|||
Loreti, E., et al., Glucose and disaccharide-sensing mechanisms modulate the expression of α-amylase in barley embryos. Plant Physiol. 123, 939-948, (2000)</ref><ref>http://www.sigmaaldrich.com/catalog/search/ProductDetail/SIGMA/T2754</ref> |
|||
==References== |
==References== |
||
{{Reflist}} |
{{Reflist}} |
||
{{carbohydrates}} |
|||
[[Category:Disaccharides]] |
[[Category:Disaccharides]] |
||
Latest revision as of 15:27, 17 June 2023
| |
| Names | |
|---|---|
| IUPAC name
α-D-glucopyranosyl-(1→3)-α-D-fructofuranose
| |
| Systematic IUPAC name
(3S,4R,5R)-1,4,5,6-tetrahydroxy-3-[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyhexan-2-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.008.108 |
| MeSH | turanose |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H22O11 | |
| Molar mass | 342.30 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Turanose is a reducing disaccharide. The d-isomer is naturally occurring. Its systematic name is α-d-glucopyranosyl-(1→3)-α-d-fructofuranose. It is an analog of sucrose not metabolized by higher plants, but rather acquired through the action of sucrose transporters for intracellular carbohydrate signaling. In addition to its involvement in signal transduction, d-(+)-turanose can also be used as a carbon source by many organisms including numerous species of bacteria and fungi.[2][3][4][5][6]
References[edit]
- ^ Turanose - Compound Summary, PubChem
- ^ Sinha, A.K.; et al. (2002). "Metabolizable and non-metabolizable sugars activate different signal transduction pathways in tomato". Plant Physiol. 128 (4): 1480–1489. doi:10.1104/pp.010771. PMC 154275. PMID 11950996.
- ^ Gonzali, S.; et al. (2005). "A turanose-insensitive mutant suggests a role for WOX5 in auxin homeostasis in Arabidopsis thaliana". Plant J. 44 (4): 633–645. doi:10.1111/j.1365-313X.2005.02555.x. PMID 16262712.
- ^ Sivitz, A.B.; et al. (2007). "Arabidopsis sucrose transporter AtSUC9. High-affinity transport activity, intragenic control of expression, and early flowering mutant phenotype". Plant Physiol. 143 (1): 188–198. doi:10.1104/pp.106.089003. PMC 1761979. PMID 17098854.
- ^ Loreti, E.; et al. (2000). "Glucose and disaccharide-sensing mechanisms modulate the expression of α-amylase in barley embryos". Plant Physiol. 123 (3): 939–948. doi:10.1104/pp.123.3.939. PMC 59056. PMID 10889242.
- ^ D-Turanose at Sigma-Aldrich
