Treprostinil
This article needs additional citations for verification. (October 2021) |
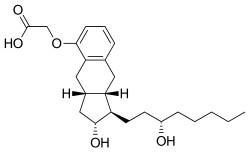 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Remodulin, Orenitram, Tyvaso, others |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Subcutaneous, intravenous, inhalation, by mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~100% |
| Metabolism | Substantially metabolized by the liver |
| Elimination half-life | 4 hours |
| Excretion | Urine (79% of administered dose is excreted as 4% unchanged drug and 64% as identified metabolites); feces (13%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.236.149 |
| Chemical and physical data | |
| Formula | C23H34O5 |
| Molar mass | 390.520 g·mol−1 |
| |
| | |
Treprostinil, sold under the brand names Remodulin for infusion, Orenitram for oral, and Tyvaso for inhalation, is a vasodilator that is used for the treatment of pulmonary arterial hypertension.[6]
Treprostinil was approved for use in the United States in May 2002.[7]
Medical uses
Treprostinil is indicated for the treatment of pulmonary arterial hypertension in people with NYHA Class II-IV symptoms to diminish symptoms associated with exercise.[1]
Adverse effects
- Since treprostinil is a vasodilator, its antihypertensive effect may be compounded by other medications that affect the blood pressure, including calcium channel blockers, diuretics, and other vasodilating agents.[8]
- Because of treprostinil's inhibiting effect on platelet aggregation, there is an increased risk of bleeding, especially among patients who are also taking anticoagulants.[8]
- It is not known whether treprostinil is excreted in breast milk. Caution is advised when administering this medication to nursing women.
- Caution is advised when administering treprostinil to patients who have impaired kidney or liver function.[8]
Common side effects depending on route of administration:
- 85% of patients report pain or other reaction at the infusion site.[8]
Administration
For infusion
Treprostinil may be administered as a continuous subcutaneous infusion or continuous intravenous infusion.[1]
Inhaled form
The inhaled form of treprostinil was approved by the FDA in July 2009, and is sold under the brand name Tyvaso.[3][4]
Oral form
The oral form of treprostinil was approved by the FDA in December 2013, and is sold under the brand name Orenitram.[2]
History
During the 1960s a UK research team, headed by Professor John Vane began to explore the role of prostaglandins in anaphylaxis and respiratory diseases. Working with a team from the Royal College of Surgeons, Vane discovered that aspirin and other oral anti-inflammatory drugs worked by inhibiting the synthesis of prostaglandins. This finding opened the door to a broader understanding of the role of prostaglandins in the body.
Vane and a team from the Wellcome Foundation had identified a lipid mediator they called “PG-X,” which inhibited platelet aggregation. PG-X, which later would become known as prostacyclin, was 30 times more potent than any other known anti-aggregatory agent.[citation needed]
By 1976, Vane and fellow researcher Salvador Moncada published the first paper on prostacyclin, in the scientific journal Nature.[9]
Treprostinil (Remodulin) was approved for use in the United States in May 2002,[1][7] and again in July 2018.[10] Tyvaso, the inhaled form of treprostinil, was approved for use in the United States in July 2009.[11] Orenitram was approved in December 2013.[12]
Treprostinil (Trepulmix) was approved for use in the European Union in April 2020.[5]
Research
Treprostinil therapy may be effective in treating Degos disease.[13]
References
- ^ a b c d "Remodulin- treprostinil injection, solution; Sterile diluent for remodulin- water injection, solution". DailyMed. 9 October 2023. Retrieved 21 May 2024.
- ^ a b "Orenitram- treprostinil tablet, extended release; Orenitram- treprostinil kit". DailyMed. 7 November 2023. Retrieved 21 May 2024.
- ^ a b "Tyvaso- treprostinil inhalant". DailyMed. 8 December 2023. Retrieved 21 May 2024.
- ^ a b "Tyvaso DPI- treprostinil inhalant; Tyvaso DPI- treprostinil kit". DailyMed. 26 January 2024. Retrieved 21 May 2024.
- ^ a b "Trepulmix EPAR". European Medicines Agency (EMA). 29 January 2020. Retrieved 9 April 2020.
- ^ Torres F, Rubin LJ (January 2013). "Treprostinil for the treatment of pulmonary arterial hypertension". Expert Review of Cardiovascular Therapy. 11 (1): 13–25. doi:10.1586/erc.12.160. PMID 23259441. S2CID 29661141.
- ^ a b "Drug Approval Package: Remodulin (Treprostinil Sodium) NDA #021272". U.S. Food and Drug Administration (FDA). 24 December 1999. Retrieved 9 April 2020.
- ^ a b c d Kumar P, Thudium E, Laliberte K, Zaccardelli D, Nelsen A (December 2016). "A Comprehensive Review of Treprostinil Pharmacokinetics via Four Routes of Administration". Clinical Pharmacokinetics. 55 (12): 1495–1505. doi:10.1007/s40262-016-0409-0. PMC 5107196. PMID 27286723.
- ^ Moncada S, Gryglewski R, Bunting S, Vane JR (October 1976). "An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation". Nature. 263 (5579): 663–665. Bibcode:1976Natur.263..663M. doi:10.1038/263663a0. PMID 802670. S2CID 4279030.
- ^ "Drug Approval Package: Remodulin". U.S. Food and Drug Administration (FDA). 7 February 2019. Retrieved 9 April 2020.
- ^ "Drug Approval Package: Tyvaso (Treprostinil) Inhalation Solution NDA #022387". U.S. Food and Drug Administration (FDA). 24 December 1999. Retrieved 9 April 2020.
- ^ "Drug Approval Package: Orenitram (Treprostinil) Extended Release Tablets NDA #203496". U.S. Food and Drug Administration (FDA). 24 December 1999. Retrieved 9 April 2020.
- ^ Shapiro LS, Toledo-Garcia AE, Farrell JF (April 2013). "Effective treatment of malignant atrophic papulosis (Köhlmeier-Degos disease) with treprostinil--early experience". Orphanet Journal of Rare Diseases. 8: 52. doi:10.1186/1750-1172-8-52. PMC 3636001. PMID 23557362.
Further reading
- Narine L, Hague LK, Walker JH, Vicente C, Schilz R, Desjardins O, et al. (December 2005). "Cost-minimization analysis of treprostinil vs. epoprostenol as an alternate to oral therapy non-responders for the treatment of pulmonary arterial hypertension". Current Medical Research and Opinion. 21 (12): 2007–2016. doi:10.1185/030079905X75104. PMID 16368052. S2CID 13162585.
{{cite journal}}: CS1 maint: overridden setting (link)
