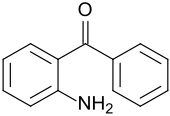
2-aminobenzophenone
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 2-aminobenzophenone | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 13 H 11 NO | |||||||||||||||
| Brief description |
yellow crystalline solid or pale to dark yellow crystal powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 197.24 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density | ||||||||||||||||
| Melting point | ||||||||||||||||
| boiling point |
220 ° C (with decomposition) |
|||||||||||||||
| solubility |
practically insoluble in water, soluble in methanol and dimethyl sulfoxide |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
In addition to an aromatic ketone group, 2-aminobenzophenone has an amino group and is used as a starting material for many benzo-fused heterocycles , as well as for pesticides , pharmaceutical drugs and pigments .
Occurrence and representation
In 1894, Carl Graebe and Fritz Ullmann reported the synthesis of o-aminobenzophenone by Hofmann rearrangement of o-benzoylbenzoic acid amide with sodium hypobromite formed from bromine in an alkaline medium . A variant of Hofmann degradation with sodium hypochlorite comes from Hans-Jürgen Quadbeck-Seeger and co-workers , whereby 2-aminobenzophenone is obtained in 98% yield.
A three-step synthesis of 2-benzoylaniline, starting from 2-nitrobenzyl chloride , was described shortly afterwards by Siegmund Gabriel .
In a Friedel-Crafts alkylation , 2-nitrobenzyl chloride is reacted with benzene , the methylene group is oxidized to the ketone group with chromium trioxide CrO 3 and the aromatic nitro group is reduced to the amine with tin (II) chloride SnCl 2 . The total yield over all stages was approx. 54%.
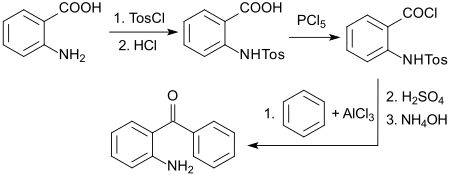
A standard process starts from inexpensive anthranilic acid which, after protecting the amino group with tosyl chloride , is reacted with phosphorus pentachloride to form the acid chloride , which reacts with benzene in a Friedel-Crafts acylation to form the protected benzophenone.
The tosyl group is treated with strong acids such as. B. concentrated sulfuric acid or hydrochloric acid and the amino function is released with ammonium hydroxide . The overall yield is 54%.
A number of alternative routes are described in the literature, but these have not been implemented because of expensive reactants, multi-stage reaction sequences and low yields.
properties
2-aminobenzophenone is a yellow crystalline solid that is practically insoluble in water and dissolves in methanol, hot ethanol and DMSO.
Applications
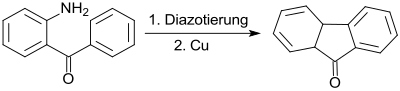
The diazonium salt of 2-aminobenzophenone can be cyclized with copper in a Pschorr cyclization to give fluorenone .
Benzo-fused heterocycles from 2-aminobenzophenone
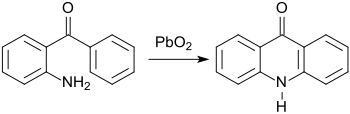
The preparation of benzo-fused nitrogen heterocycles from 2-aminobenzophenone was already described in early work by C. Graebe and S. Gabriel, e.g. B. the acridine derivative acridone by oxidation with lead (IV) oxide PbO 2
and the quinazoline derivative 4-phenylquinazolin-2-one by reaction with urea .
Positional isomeric acridones are used in the acid-catalyzed reaction of 2-aminobenzophenone with 1,3-diketones, such as. B. obtained 1,3-cyclohexanedione .
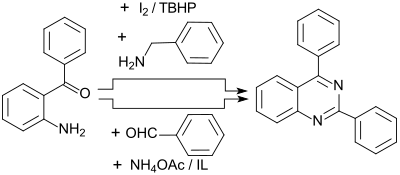
Oxygen-free quinazolines are in high yields of up to 95% by reaction of 2-aminobenzophenone with benzylamines and oxidation with iodine / tert-butyl hydroperoxide I 2 / TBHP
or with benzaldehydes and ammonium acetate in the presence of ionic liquids (engl. ionic liquids IL) accessible. The 1,2-dihydroquinazolines formed as intermediates are also oxidized to quinazolines by atmospheric oxygen.
Recently, nitrogen-containing benzo-fused heterocycles with three nitrogen atoms, such as 1,2,3-benzotriazines, have also been synthesized via the corresponding hydrozone and its oxidation with lead (IV) acetate .
Pharmacologically active ingredients from 2-aminobenzophenone
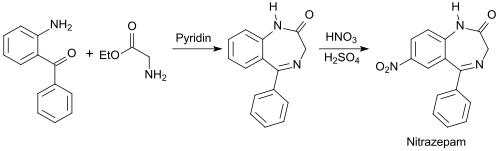
2-Benzoylaniline is used as a starting material for benzodiazepines , such as. B. Nitrazepam Mogadan R used,
while the production of chlordiazepoxide Librium R starts from 2-amino-5-chlorobenzophenone.
The antihistamine epinastine can be obtained in a multi-step synthesis from 2-aminobenzophenone.
Pigments made from 2-aminobenzophenone
The reaction of diethyl succinylosuccinate with 2-aminobenzophenone produces a pale yellow condensation product in an acid-catalyzed Friedlaender condensation, which is oxidized with chloranil to form a violet intermediate which, when heated, gives a metallic green insoluble pigment.
One reaction variant subjects the soluble condensation product directly to an oxidative cyclization at high temperature (255 ° C.), the tetrabenzodiazaketoperylene pigment being obtained as a black crystalline solid in 95% yield. Pigment preparations of the compound show light-stable dark purple to black shades.
2-aminobenzophenone reacts with 1,2-diiodobenzene in a Ullmann reaction in the presence of potassium carbonate and copper to form a yellow dibenzo phenanthroline pigment.
Individual evidence
- ↑ a b data sheet 2-aminobenzophenone from Sigma-Aldrich , accessed on March 15, 2019 ( PDF ).
- ↑ a b c Entry on 2-aminobenzophenones at TCI Europe, accessed on March 15, 2019.
- ^ Carl L. Yaws: Thermophysical Properties of Chemicals and Hydrocarbons, 2nd Edition . Elsevier Inc., Amsterdam, NL 2016, ISBN 978-0-323-28659-6 , pp. 322 .
- ↑ a b c data sheet 2-aminobenzophenone at AlfaAesar, accessed on March 15, 2019 ( PDF )(JavaScript required) .
- ^ William M. Haynes: CRC Handbook of Chemistry and Physics, 97th Edition . CRC Press, Boca Raton, FL, USA 2016, ISBN 978-1-4987-5429-3 , pp. 3-32 .
- ↑ a b c C. Graebe, F. Ullmann: Presentation of o-aminobenzophenone and synthesis of acridone . In: Ber. German Chem. Ges. Volume 27 , no. 3 , 1894, pp. 3483-3484 , doi : 10.1002 / cber.189402703170 .
- ↑ C. Graebe, F. Ullmann: About o-aminobenzophenon . In: Liebigs Ann. Chem. Band 291 , no. 1−2 , 1896, pp. 8-16 , doi : 10.1002 / jlac.18962910103 .
- ↑ Patent US4082749 : Process for the production of amines. Registered on June 3, 1974 , published on April 4, 1978 , applicant: BASF AG, inventor: H.-J. Quadbeck-Seeger.
- ^ A b S. Gabriel, R. Stelzner: On the knowledge of the quinazoline compounds . In: Ber. German Chem. Ges. Volume 29 , no. 2 , 1896, p. 1300-1316 , doi : 10.1002 / cber.18960290226 .
- ^ HJ Scheifele, Jr., DF DeTar: 2-aminobenzophenone In: Organic Syntheses . 32, 1952, p. 8, doi : 10.15227 / orgsyn.032.0008 ; Coll. Vol. 4, 1963, p. 34 ( PDF ).
- ↑ Patent US6310249B1 : Process for producing 2-aminobenzophenone compound. Applied July 22, 1999 , published October 30, 2001 , applicant: Nissan Chemical Industries , Ltd., inventor: H. Matsumoto, T. Horiuchi.
- ^ DA Walsh: The Synthesis of 2-Aminobenzophenones . In: Synthesis . tape 9 , 1980, pp. 677-688 , doi : 10.1055 / s-1980-29169 .
- ↑ LJ Kumar, S. Sarveswari, V. Vijayakumar: DMFDMA catalyzed synthesis of 2 - ((dimethylamino) methylene) -3,4-dihydro-9arylacridin-1 (2H) -ones and their derivatives: invitro antifungal, antibacterial and antioxidant evaluations . In: Open Chem. Band 16 , 2018, p. 1077-1088 , doi : 10.1015 / chem-2018-0110 .
- ↑ J. Zhang, D. Zhu, C. Yu, C. Wan, Z. Wang: A simple and efficient approach to the synthesis of 2-phenylquinazolines via sp 3 C – H functionalization . In: Org. Lett. tape 12 , no. 12 , 2010, p. 2841-2843 , doi : 10.1021 / ol100954x .
- ↑ SK Panja, S. Saha: Recyclable, magnetic ionic liquid bmim [FeCl 4 ] -catalyzed, multicomponent, solvent-free, green synthesis of quinazolines . In: RSC Adv. Band 3 , 2013, p. 14495-14500 , doi : 10.1039 / c3ra42039f .
- ↑ C. Derabli, R. Boulcina, G. Kirsch, B. Carboni, A. Debache: A DMAP-catalyzed mild and efficient synthesis of 1,2-dihydroquinazolines via a one-pot three-component protocol . In: Tetrahedron Lett. tape 55 , no. 1 , 2014, p. 200-204 , doi : 10.1016 / j.tetlet.2013.10.157 .
- ↑ BM Adger, S. Bradbury, M. Keating, CW Rees, RC Storr, MT Williams: 1,2,3-Benzotriazines . In: J. Chem. Soc., Perkin Trans. 1 . tape 0 , no. 1 , 1975, p. 31-40 , doi : 10.1039 / P19750000031 .
- ↑ Patent DE2252378 : Process for the production of benzodiazepine derivatives. Registered on October 25, 1972 , published on May 24, 1973 , applicant: F. Hoffmann-La Roche & Co., AG, inventor: H. Boemches, H. Meyer.
- ↑ Patent US2893992 : 1,4-Benzodiazepine 4-oxides. Registered on May 15, 1958 , published July 7, 1959 , applicant: F. Hoffmann-La Roche Inc., inventor: Leo Sternbach .
- ↑ Patent CN103012408A : Synthesis method of epinastin. Applied on November 28, 2012 , published on April 3, 2013 , Applicant: Guangzhou Inst. Biomed & Health, Inventor: Q. Cai, J. Liu.
- ↑ K. Kitahara, H. Nishi: New heterocyclic compounds derived from diethyl 2,5 ‐ dioxo ‐ 1,4 ‐ cyclohexanedicarboxylate and 2 ‐ aminobenzophenone . In: J. Heterocycl. Chem. Band 25 , no. 4 , 1988, pp. 1063-1065 , doi : 10.1002 / jhet.5570250403 .
- ↑ Patent US5028643 : Tetrabenzodiazaketoperylene pigment. Applied on June 27, 1989 , published July 2, 1991 , applicant: Ciba-Geigy Corp., inventor: EE Jaffe.
- ↑ D. Hellwinkel, P. Ittemann: A general synthesis method for dibenzo [b, j] [x, z] phenanthrolines with x, z = 1.7; 4.7 and 1.10 . In: Liebigs Ann. Chem. Band 1985 , no. 7 , 1985, pp. 1501-1507 , doi : 10.1002 / jlac.198519850722 .