4-fluorobenzonitrile
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 4-fluorobenzonitrile | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 7 H 4 FN | |||||||||||||||
| Brief description |
colorless or light yellow to yellow crystal lumps or needles |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 121.11 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density | ||||||||||||||||
| Melting point | ||||||||||||||||
| boiling point |
188 ° C at 1000 hPa |
|||||||||||||||
| solubility |
Slightly soluble in water, soluble in toluene , dimethyl sulfoxide and in most organic solvents |
|||||||||||||||
| Refractive index |
1.4925 (55 ° C, 589 nm) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
In addition to an aromatic nitrile group, 4-fluorobenzonitrile has a fluorine atom in the 4-position ( para position) and is used as a raw material for pesticides , active pharmaceutical ingredients , liquid crystals and pigments .
Occurrence and representation
For 4-fluorobenzonitrile a variety of synthetic routes have been developed which, in the halogen exchange (Cl, Br, I → F) exchanging halogen for nitrile (Br → CN) or the conversion of a para -ständigen carbonyl or carboxyl group to cyano group is based .
4-fluorobenzonitrile by halogen exchange
Thus, p -fluorobenzonitrile is formed by exchanging Cl for F in the sense of a nucleophilic aromatic substitution on an electron-poor aromatic, such as 4-chlorobenzonitrile, with potassium fluoride KF in 1,3-dimethyl-2-imidazolidinone DMEU at 290 ° C in a pressure-tight reactor in 91% yield.
Starting from 4-bromofluorobenzene , which can be obtained very efficiently from fluorobenzene with bromine in the presence of catalytic amounts of iron (III) chloride at low temperatures (> 97% yield with> 99% purity), 4-fluorobenzonitrile can be prepared by reaction with acetone cyanohydrin in the presence of palladium (II) acetate Pd (OAc) 2 , the bidentate chelate ligand 1,5-bis (diphenylphosphanyl) pentane dpppe and the diamine tetramethylethylenediamine TMEDA (98% yield).
In a similar way, PFBN is formed from 4-bromofluorobenzene with sodium cyanide as the cyan source with palladium catalysis (Pd 0 / t-Bu 3 P) in an acetonitrile - THF mixture in 97% yield.
4-fluorobenzonitrile from 4-fluorobenzaldehyde
4-fluorobenzaldehyde - obtainable from 4-chlorobenzaldehyde by halogen exchange in 75% yield - can be oxidized directly to 4-cyanofluorobenzene in a yield of 92% in aqueous ammonia with 2-iodoxybenzoic acid IBX via the imine formed as an intermediate .
In a one-pot reaction in the “green” solvent glycerine , 4-fluorobenzaldehyde reacts with hydroxylamine hydrochloride at 90 ° C via the intermediate oxime to form 4-fluorobenzonitrile in 83% yield. In DMSO as a solvent, PFBN is obtained in 95% yield.
4- fluorobenzonitrile from 4- fluorobenzamide
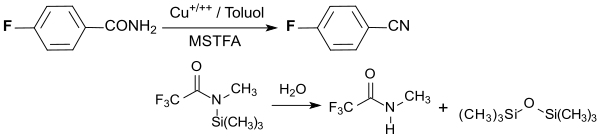
The conversion of a para carboxyl group into a nitrile group proceeds quantitatively by dehydration of 4-fluorobenzamide with a copper salt in toluene at 100 ° C with the silylation reagent N-methyl-N- (trimethylsilyl) trifluoroacetamide MSTFA CF 3 CON (CH 3 ) Si ( CH 3 ) 3 .
N-methyltrifluoroacetamide CF 3 CONHCH 3 and hexamethyldisiloxane (CH 3 ) 3 SiOSi (CH) 3 are produced as by-products .
4-fluorobenzonitrile from other starting materials
Further alternative routes with other reactants are described in the literature, e.g. B. from 4-fluorobenzyl alcohol by oxidation with the nitroxide radical TEMPO to 4-fluorobenzaldehyde and its reaction with iodine I 2 and aqueous ammonia NH 3 in 99% yield.
properties
Pure 4-fluorobenzonitrile is a white to yellow crystalline solid that does not dissolve very much in water, but in many organic solvents. The odor of PFBN is described as unpleasant.
Applications
The synthesis of diaryl ethers and thioethers with para cyano groups succeeds practically quantitatively by reacting 4-fluorobenzonitrile with substituted phenols in acetonitrile in the presence of potassium fluoride on basic aluminum oxide and the crown ether 18-crown-6 .
The diaryl ether of PFBN with the sodium salt of p-cresol and subsequent hydrogenation of the cyano to aminomethyl group provides the variable part of the molecule of the pyrazole - insecticide tolfenpyrad .
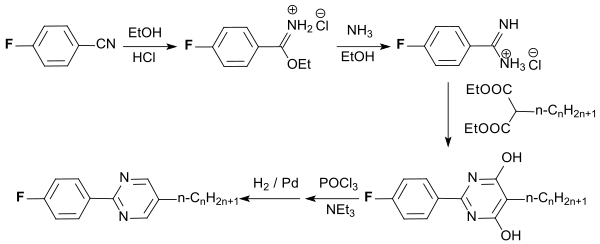
Rod-shaped liquid-crystalline fluorophenylpyrimidines with high chemical and thermal stability and light resistance can be obtained from 4-fluorobenzonitrile by multistage synthesis.
The compounds form low-viscosity nematic mesophases in a wide temperature range and are suitable for use in LC displays .
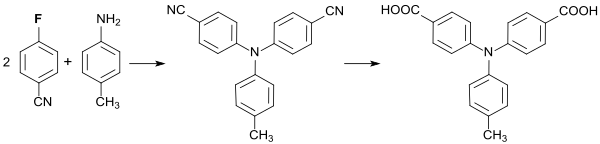
Reaction of 4-fluorobenzonitrile with p-toluidine produces 4,4'-dicyano-4 "-methyltriphenylamine, the cyano groups of which are hydrolyzed to the corresponding aromatic dicarboxylic acid and polycondensed with aromatic diamines to give polyamides .
The polymers obtained have interesting properties as blue-emitting electrochromic materials.
The s-triazine pigment 2,4,6-tris (4-fluorophenyl) -1,3,5-triazine (TFPT), which reacts with the adduct formed from 4-fluorobenzonitrile and sulfur trioxide with SO 3 , shows intense blue fluorescence 4-fluorobenzamidine hydrochloride is accessible.
Nanoparticles containing such fluorescent triazine pigments are biocompatible and can be used for imaging in living cells.
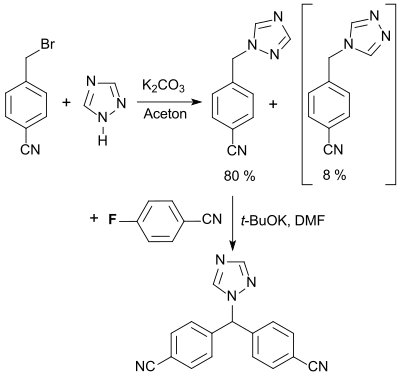
The most important use of 4-fluorobenzonitrile is as a building block for the aromatase inhibitor letrozole (Femara R ), which is used to treat breast cancer in postmenopausal women.
The proposed synthesis and work-up process is intended to provide letrozole in high yield and purity, with as little contamination as possible from the 1,3,4-triazolyl isomer formed in the first step of the reaction with 1,2,4-triazole .
Individual evidence
- ↑ a b c d e f data sheet 4-fluoro-benzonitrile from Sigma-Aldrich , accessed on August 18, 2019 ( PDF ).
- ↑ Entry on 4-fluorobenzonitrile at TCI Europe, accessed on August 18, 2019.
- ↑ a b c d William M. Haynes: CRC Handbook of Chemistry and Physics, 96th Edition . CRC Press, Boca Raton, FL, USA 2015, ISBN 978-1-4822-6097-7 , pp. 3-274 .
- ↑ a b R.RP Torregrosa: 4-fluorobenzonitrile . In: e-EROS Encyclopedia of Reagents for Organic Synthesis . 2015, doi : 10.1002 / 047084289X.rn01850 .
- ↑ a b S.RK Samala, K. Gokavarapu, SB Rao, S. Gokavarapu, S. Gandhi: A mild, simple, cost efficient, chemoselective, and high yielding procedure for the anti-cancer drug letrozole key intermediate 4-fluorobenzonitrile . In: J. Current Res. Band 10 , no. 08 , 2018, p. 72908–72910 , doi : 10.24941 / ijcr.32211.08.2018 .
- ↑ H. Suzuki, Y. Kimura: Synthesis of 3,4-difluorobenzonitrile and monofluorobenzonitriles by means of halogen-exchange fluorination . In: J. Fluor. Chem. Band 52 , no. 3 , 1991, pp. 341-351 , doi : 10.1016 / S0022-1139 (00) 80348-6 .
- ↑ Patent US5847241 : Process for the preparation of p-bromofluorobenzene. Applied on August 26, 1996 , published December 8, 1998 , Applicant: Bromine Compounds Ltd., Inventor: J. Oren.
- ↑ M. Sundermeier, A. Zapf, M. Beller: A practicable process for the palladium-catalyzed cyanation of aryl halides . In: Angew. Chem. Band 115 , no. 14 , 2003, p. 1700–1703 , doi : 10.1002 / anie.200250778 .
- ↑ AV Ushkov, VV Grushin: Rational catalysis design on the basis of mechanistic understanding: Highly efficient Pd-catalyzed cyanation of aryl bromides with NaCN in recyclable solvents . In: J. Amer. Chem. Soc. tape 133 , no. 28 , 2011, p. 10999–11005 , doi : 10.1021 / ja2042035 .
- ^ WL McEwen: p-Chlorobenzaldehyde In: Organic Syntheses . 11, 1932, p. 12, doi : 10.15227 / orgsyn.012.0012 ; Coll. Vol. 2, 1943, p. 133 ( PDF ).
- ↑ Y. Yoshida, Y. Kimura: A convenient synthesis of fluorobenzaldehyde by KF / Ph 4 PBr / 18-crown-6 reagent system . In: Chem. Lett. tape 17 , no. 8 , 1988, pp. 1355-1358 , doi : 10.1246 / cl.1988.1355 .
- ↑ ND Arote, DS Bhalerao, KG Akamanchi: Direct oxidative conversion of aldehydes to nitriles using IBX in aqueous ammonia . In: Tetrahedron Lett. tape 48 , no. 21 , 2007, p. 3651-3653 , doi : 10.1016 / j.tetlet.2007.03.137 .
- ↑ AP Ingale, SM Patil, SV Shinde: Catalyst-free, efficient and one pot protocol for synthesis of nitriles from aldehydes using glycerol as green solvent . In: Tetrahedron Lett. tape 58 , no. 52 , 2017, p. 4845-4848 , doi : 10.1016 / j.tetlet.2017.11.032 .
- ↑ S. Enthaler, M. Weidauer: Copper-ctalyzed dehydration of primary amides to nitriles . In: Catal. Lett. tape 141 , 2014, pp. 1079-1085 , doi : 10.1007 / s10562-011-0660-9 .
- ↑ H. Shimojo, K. Moriyama, H. Togo: Simple one-pot conversion of alcohols into nitriles . In: Synthesis . tape 45 , no. 15 , 2013, p. 2155-2164 , doi : 10.1055 / s-0033-1338489 .
- ↑ JS Sawyer, EA Schmittling, JA Palkowitz, WJ Smith: Synthesis of diaryl ethers, diaryl thioethers, and diarylamines mediated by potassium fluoride - alumina and 18-crown-6: Expansion of scope and utility . In: J. Org. Chem. Band 63 , no. 18 , 1998, pp. 6338-6343 , doi : 10.1021 / jo980800g .
- ↑ W. Krämer, U. Schirmer, P. Jeschke, M. Witschel (Eds.): Modern Crop Protection Compounds: 3 Volume Set . Wiley-VCH, Weinheim 2011, ISBN 978-3-527-32965-6 , pp. 1087 .
- ↑ Patent EP0126949A2 : Fluorine-containing pyrimidine derivatives. Applied on April 17, 1984 , published on December 5, 1984 , Applicant: Merck Patent GmbH, Inventors: J. Krause, M. Römer, L. Pohl, B. Scheuble, G. Weber.
- ↑ G.-S. Liou, N.-K. Huang, Y.-L. Yang: Blue-light-emitting and anodically electrochromic materials of new wholly aromatic amides derived from the high-efficiency chromophore 4,4'-dicarboxy-4 "-methyltriphenylamine . In: J. Polym. Sci. Pole. Chem. Band 44 , no. 13 , 2006, p. 4095-4107 , doi : 10.1002 / pola.21505 .
- ↑ K. Zhang, K. Yang, S. Ai, J. Xu: Synthesis of novel cross-linked s-triazine-containing poly (aryl ether) s nanoparticles for biological fluorescent labeling . In: Des. Monomers Polym. tape 20 , no. 1 , 2016, p. 389-396 , doi : 10.1080 / 1685551.2017.1281786 .
- ↑ Patent US20100234617A1 : Process for preparation of letrozole and its intermediates. Applied on January 16, 2008 , published on September 16, 2010 , Applicant: Fresenius Kabi Oncology, Ltd., Inventor: VK Shrawat, JP Singh, RP Nautiyal.