Apoptosis
The apoptosis ( ancient Greek ἀπόπτωσις apoptosis of ἀποπίπτειν apopíptein , fall off ') is a form of programmed cell death . It is a "suicide program" of individual biological cells . This can be stimulated externally (e.g. by immune cells ) or triggered by internal cell processes (e.g. after severe damage to the genetic information ). In contrast to the other important mechanism of cell death, necrosis , apoptosis is actively carried out by the cell itself and is therefore part of the cell's metabolism . As a result, this form of cell death is subject to strict control and it is ensured that the cell in question perishes without damaging the neighboring tissue.
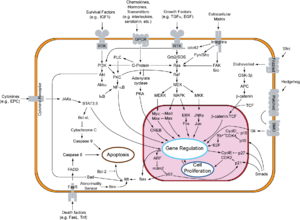
In contrast to the other forms of programmed cell death, proteolytic enzymes , so-called caspases , play a central role in apoptosis .
Apoptosis and necrosis are usually easy to distinguish optically: While apoptosis begins to shrink and the DNA is broken down into defined pieces by endonucleases (known as the DNA ladder and detectable by means of electrophoresis and the so-called TUNEL method ), swells with the Necrosis affects the cell, destroying its plasma membrane. As a result, local inflammation occurs, as cytoplasm and cell organelles are released into the extracellular space , which must be eliminated by macrophages (phagocytes). Compared to necrosis, apoptosis is the more common form of cell death. In certain cases, however, apoptosis and necrosis cannot be clearly separated from one another. The transition between the two forms of cell death is then fluid and is called aponecrosis .
The German-Swiss naturalist Carl Vogt was the first to discover apoptosis in 1842 while studying the development of tadpoles of the common midwife toad . The great importance of this discovery was (only) recognized over 100 years later. 1972 coined John FR Kerr , Andrew Wyllie and Alastair R. Currie of the University of Aberdeen the term apoptosis' (English apoptosis ).
Occurrence
During the development of an organism, apoptosis is essential:
- During the metamorphosis from the tadpole to the frog or the degeneration of the skins between the fingers / toes (interdigital skins), cells are specifically stimulated to apoptosis
- through apoptotic cell death of the cells of the vitreous humor and lens of the lens eye , the light permeability of the eye lens is achieved
- To ensure the correct “interconnection” of brain structures and individual nerve cells, up to half of all originally formed nerve cells die again before birth
But it is also essential in the adult organism:
- to control the number of cells and the size of tissues
- in the rejuvenation of tissues (e.g. in the olfactory epithelium of the nose)
- in the selection and breakdown of unnecessary or potentially harmful cells of the immune system
- to eliminate degenerate cells
- to ensure plasticity in the central nervous system
- for the selection of germ cells (approx. 95 percent of the germ cells are apoptotically killed before they reach maturity)
- in holocrine secretion , d. H. in the sebum glands of humans
Currently, apoptosis is particularly related to the cancer genesis and various autoimmune diseases studied. One goal of cancer research is to trigger controlled apoptosis in degenerated cells. But cancer cells also use the apoptosis mechanism to switch off human defense cells, so-called tumor-infiltrating lymphocytes (TILs). An apoptosis-inducing protein, the CD95 ligand ( Fas ligand ), can be found on the surface of various tumor cell lines . This mechanism is known as tumor counterattack .
The question of the role of apoptosis in neurodegenerative diseases (such. As Alzheimer's disease , Huntington's disease , Parkinson's disease , ALS plays) is also being hotly debated, and run in this field various researches.
Signs of apoptosis have also been found in unicellular organisms. In Saccharomyces cerevisiae (baker's yeast, brewer's yeast), various markers of apoptosis (DAPI, TUNEL staining ) become visible , especially in old cells . There is speculation about evolutionary reasons for the existence of apoptosis in single cells. One theory says that individual damaged cells sacrifice themselves and commit "suicide" for the benefit of the collective. This saves nutrients, which are then available to the other cells. Ultimately, the goal is to preserve the genome, which is practically identical in the other cells.
presentation
histology
The course of apoptosis can be followed with a light microscope . First, the cell in question detaches itself from the tissue association. In the further course the cell stains more and more eosinophil and becomes increasingly smaller. In addition, visible vesicles form on the cell membrane. The cell nucleus becomes smaller and more densely packed. It can also break down into several parts in the course of apoptosis. At the end of the process, a homogeneous eosinophilic apoptosis body remains. This is then broken down by phagocytosis . The programmed cell death does not trigger an inflammatory reaction.
Imaging procedures
Apoptosis can be by means of imaging techniques such as positron emission tomography , fluorescence imaging ( fluorescence imaging ) and magnetic resonance imaging macroscopically in vivo demonstrated ( molecular imaging ). As tracer , modified amino acids , such as (5-dimethylamino) -1-napththalinsulfonyl-α-ethyl-fluoroalanine (NST-732) or N , N '-Didansyl- L -cystin used.
Signal transduction pathways
The process of apoptosis can be divided into two phases: initiation and effector phase.
Initiation phase
As a rule, a distinction is made between two processes with regard to the initiation phase: the extrinsic (type I) and the intrinsic (type II) path. A strict separation of the processes is hardly possible in vivo .
Extrinsic way - Type I.
The extrinsic pathway is initiated by ligand binding to a receptor of the TNF receptor family (z. B. CD95). These so-called death receptors have a death domain (DD, "death domain") in their cytoplasmic part. Ligands are, for example, tumor necrosis factor (TNF) and other cytokines that are secreted, for example, by T lymphocytes .
As a result of the induced trimerization of the receptor, the death domains form a structure to which adapter molecules with their own death domain can now bind through homotypic interactions. In a first step, the "TNF receptor-associated protein" (TRADD) is recruited. The “Fas-associated protein with death domain” (FADD) then binds to the DD of the TRADD. In addition to the DD, FADD also has a death effector domain (DED) through which the proCaspase 8 and its DED binds to the complex. This can now activate itself autocatalytically due to the high local concentration that has arisen. The active caspase 8 in turn triggers the so-called caspase cascade, which activates further caspase 8 molecules in a signal-amplifying feedback.
In AIDS patients , for example , numerous uninfected leukocytes also die through this mechanism : the HI virus uses the protein Nef to stimulate immune cells that have not yet been diseased to programmed cell death. The inhibitor Fasudil can stop this mechanism.
A lack of contact with the extracellular matrix also makes cells apoptotic. This process is known as anoikis .
Intrinsic Path - Type II
In the intrinsic pathway or apoptosis of type II, mechanisms that are not yet precisely known lead to the release of cytochrome c and other pro-apoptotic factors such as Smac / DIABLO from the mitochondria into the cytoplasm. This pathway can be triggered by tumor suppressors such as p53 , a transcription factor that is activated when DNA is damaged . p53 stimulates the expression of pro-apoptotically acting members of the Bcl-2 family (e.g. Bax , Bad ). These then lead to the release of pro-apoptotic factors - such as cytochrome c - from the mitochondrial intermembrane space. However, many toxic substances act, such as. B. chemotherapeutic agents, also directly on the mitochondria and can thus induce type II apoptosis. The binding of cytochrome c and dATP to Apaf-1 (apoptotic protease activating factor-1) causes a conformational change of the protein. This change in conformation makes the protein binding domain CARD (caspase recruitment domain) of Apaf-1 accessible, so that it can bind to the CARD domain of procaspase 9. The formation of this heterodimer is a prerequisite for the autolytic activation of caspase 9. This complex is called the apoptosome and represents the active form of caspase 9. Analogous to caspase 8, active caspase 9 initiates the caspase cascade. A signal amplification of this pathway is mediated within the caspase cascade by caspase 7, which not only cleaves substrates that are involved in the execution of apoptosis, but also activates caspase 9.
Cells that may not be able to initiate type I apoptosis due to an insufficient intracellular amount of caspase 8 can activate the mitochondrial pathway for signal amplification. For this purpose, caspase 8 cleaves the cytosolic protein Bid (“BH3 interacting domain death agonist”). The resulting C-terminal cleavage product tBid (“truncated bid”) mediates the release of pro-apoptotic factors after translocation into the mitochondria and leads to the activation of caspase 9.
Stress-induced path - (Type III)
Stress reactions of the endoplasmic reticulum , which can be caused, for example, by deregulated depletion of the ER calcium store, glucose deficiency, hypoxia or misfolded proteins ( unfolded protein response ), can initiate apoptosis. There is a transcription factor and a caspase-dependent signal path.
Execution phase and caspase cascade
So-called effector caspases, primarily caspases 3, 6 and 7, lead to apoptotic death of the cell. They themselves are actively involved in the breakdown of lamin (in the cell nuclear membrane) and actin (part of the cytoskeleton ). On the other hand, they activate secondary target proteins (e.g. caspase-activated DNase , CAD, or other caspases) through limited proteolysis . The DNase cleaves genomic DNA at internucleosomal marked regions (left region) and produces 180-185 bp fragments. This characteristic length pattern can be represented in an agarose gel electrophoresis as "apoptosis ladder". The representation of the "apoptosis ladder" is therefore a sensitive method to distinguish apoptosis from ischemic or toxic cell death. Another aspect is the caspase-mediated suppression of DNA repair .
Ultimately, the cell gradually constricts in small vesicles , which in turn are taken up by specialized "scavenger cells" ( phagocytes ). In contrast to necrosis , the cell membrane remains intact.
The leakage of cytochrome c from mitochondria into the cytoplasm, which is a ubiquitous sign of apoptosis, occurs in the extrinsic route late during apoptosis and is more the result of apoptosis than its trigger.
In the extrinsic way, a further distinction is made between active (induced by activation of receptors) and passive (triggered by withdrawal of growth factors , e.g. neurotrophins ) apoptosis.
The most important proteins involved in the suppression of apoptosis are the anti-apoptotic members of the Bcl-2 family (Bcl-2 and Bcl-x L ) and the IAPs ( inhibitor-of-apoptosis proteins ), such as for example survivin . Further upstream are the protein kinase B (alternative name: Akt), e.g. B. in connection with receptors of the Trk family (see neurotrophin ) and transcription factors of the FOXO family and the transcription factor NF-κB .
Clearance
In multicellular cells, dying (apoptotic) cells are quickly and efficiently removed from specialized or specially prepared scavenger cells (phagocytes). The common concept is that the elimination of these cells takes place without inflammation or even triggers an anti-inflammatory reaction. In contrast, the elimination of necrotic cells is more likely to trigger a pro-inflammatory (pro-inflammatory) response. Not only the dying cell itself, but also the substances released during cell death, contribute to the process of eliminating the dead cells and the resulting response of the immune system.
Controlled cell death is of vital importance for the homeostasis of multicellular organisms. During permanent cell renewal, the body has to remove billions of cell corpses created by apoptosis every day. An efficient clearance of apoptotic cells is of fundamental importance, because otherwise they tend to become secondary necrotic, release intracellular components and thereby trigger inflammation and autoimmunity.
Interaction with phagocytes
In healthy, multicellular organisms, apoptotic cells are immediately taken up either by neighboring cells capable of phagocytosis or by specialized scavenger cells (phagocytes). The problem that arises from this scenario is how the specialized phagocytes manage to reach their prey cell on time, especially if they are not in the direct vicinity of the dying cells. One possibility is that dying cells secrete soluble mediators that attract the phagocytes. In the supernatant of apoptotic cells, the following substances were identified as "find-me" signals (chemoattractants):
- Lysophosphatidylcholine (LPC)
- Nucleotides
- Thrombospondin-1 (TSP-1) and its components
- Fractalkin,
- apoptotic microblebs
- Sphingosine 1 Phosphate (S1P)
- soluble IL-6 receptor (sIL-6R)
- Cross-linked dimer of the S19 ribosomal protein (dRP S19),
- Endothelial Monocyte Activating Polypeptide II (EMAP II),
- Cleavage products of human tyrosyl-tRNA synthetase (TyrRS),
- Lactoferrin
Involvement of apoptotic cells
Examination of the clearance of apoptotic cells has revealed a complex network of interaction and communication between dying cells and phagocytes. In addition to cell-cell contacts, soluble factors that are released by apoptotic cells are also involved. Many different chemoattractants have been described that organize the process of clearance of dying cells. These pleomorphic mediators are also involved in the regulation of the immune response. They determine the degree of inflammation and thus control the decision between immune activation and tolerance induction. Interestingly, physical disorders such as chronic inflammation, autoimmunity and loss of control of tumors deregulate the production and / or function of some of these factors. Therefore, "find-me" signals are promising biomarkers for various diseases and thus potential targets for future therapeutic interventions.
Apoptotic cell clearance is the final step in the removal of old, damaged, infected and dangerous cells in the tissues of multicellular organisms. The process protects the surrounding tissue as much as possible. Apoptotic cells undergo enormous morphological changes. These include contraction, membrane blebbing (bubble formation) and an apoptotic cell shape. The membrane blebbing actively contributes to the detection and uptake of dead cell bodies and to the induction of auto-reactive antibodies. Blebbing is the formation of membrane vesicles on the surface of the apoptotic cell. These vesicles are surrounded by lipids from the cytoplasmic membrane and contain parts of the contents of the dying cell. Blebs are balloon-shaped vesicles that form on the cell surface when the plasma membrane bulges. They arise in a dynamic process during apoptosis on the entire cell surface. The formation of blebs is preceded by increased hydrostatic pressure in the cell, which is caused by the actomyosin-controlled contraction of the cell. Reshaped blebs do not yet contain actin or other cytoskeletal proteins. Later, cytoskeletal precursor proteins polymerize quickly, which leads to the regression of the blebs. The process of bleb formation and regression is repeated during the process of apoptosis. In late apoptosis, individual blebs can be filled with cell organelles and condensed chromatin. Lacerated chromatin-filled blebs can act as viromimetics and contribute to the induction of anti-nuclear antibodies. Surface blebs and released membrane-coated microparticles are mostly taken up by macrophages in the immediate vicinity.
Nobel Prize in Medicine
Scientists Sydney Brenner (Great Britain), H. Robert Horvitz (USA) and John E. Sulston (Great Britain) received the Nobel Prize for Medicine in 2002 for their discoveries relating to the genetic regulation of organ development and programmed cell death .
literature
- Hubert Hug: Apoptosis: The self-destruction of the cell as a means of survival . In: Biology in Our Time . Volume 30 (3), 2000, pp. 128-135, ISSN 0045-205X .
- Stefan Grimm: Apoptosis: Programmed cell death . In: Chemistry in Our Time . Volume 37 (3), 2003, pp. 172-178, ISSN 0009-2851
- Fritz Höffeler: The machinery of apoptosis: Chronicle of a death announced . In: Biology in Our Time . Volume 34 (1), 2004, p. 1623, ISSN 0045-205X .
- MO Hengartner: The biochemistry of apoptosis . In: Nature . 407, No. 6805, October 2000, pp. 770-6. doi : 10.1038 / 35037710 . PMID 11048727 .
- J. Yuan, BA Yankner: Apoptosis in the nervous system . In: Nature . 407, No. 6805, October 2000, pp. 802-9. doi : 10.1038 / 35037739 . PMID 11048732 .
Web links
- elaborate animation on apoptosis
- Nobel Prize in Medicine and Physiology 2002
- Apoptopedia . An introduction to apoptosis research with a review and glossary.
- A. Lawen: Apoptosis - an introduction . In: BioEssays . Volume 25, 2003, pp. 888-896. doi : 10.1002 / bies.10329
- Graphs and images of apoptosis / comparison to necrosis
Individual references and sources
- ^ I. Böhm, H. Schild: Apoptosis: the complex scenario for a silent cell death. In: Molecular imaging and biology. Volume 5, Number 1, 2003 Jan-Feb, pp. 2-14, ISSN 1536-1632 . PMID 14499155 . (Review).
- ↑ L. Formigli, L. Papucci et al. a .: Aponecrosis: morphological and biochemical exploration of a syncretic process of cell death sharing apoptosis and necrosis. In: Journal of Cellular Physiology . Volume 182, Number 1, January 2000, pp. 41-49, ISSN 0021-9541 . doi : 10.1002 / (SICI) 1097-4652 (200001) 182: 1 <41 :: AID-JCP5> 3.0.CO; 2-7 . PMID 10567915 .
- ↑ C. Vogt: Investigations into the development history of the midwife toad (Alytes obstetricans). Solothurn, Switzerland: Jent & Gassmann, 1842.
- ↑ JF Kerr, AH Wyllie, AR Currie: Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. In: British journal of cancer . Volume 26, Number 4, August 1972, pp. 239-257, ISSN 0007-0920 . PMID 4561027 . PMC 2008650 (free full text). (Review).
- ^ ME Peter, AE Heufelder, MO Hengartner: Advances in apoptosis research. In: Proceedings of the National Academy of Sciences of the United States of America . Volume 94, Number 24, November 1997, pp. 12736-12737, ISSN 0027-8424 . PMID 9398063 . PMC 34166 (free full text). (Review).
- ↑ W. Böcker, H. Denk, Ph. U. Heitz, H. Moch: Pathologie, 4th edition, Munich 2008, p. 62.
- ↑ M. Damianovich et al.: ApoSense: a novel technology for functional molecular imaging of cell death in models of acute renal tubular necrosis. In: Eur J Nucl Med Mol Imaging 33, 2006, pp. 281-291. PMID 16317537 , PMC 1998881 (free full text).
- ↑ R. Aloya et al .: Molecular imaging of cell death in vivo by a novel small molecule probe. In: Apoptosis 11, 2006, pp. 2089-2101. PMID 17051335
- ↑ a b Mutschler et al .: Mutschler drug effects: textbook of pharmacology and toxicology , Wissenschaftliche Verlagsgesellschaft, 9th edition, 2008.
- ↑ Apoptosis on antibodies online (accessed August 3, 2019)
- ↑ Szegezdi E, Fitzgerald U, Samali A: Caspase-12 and ER-stress-mediated apoptosis: the story so far . In: Ann. NY Acad. Sci. . 1010, December 2003, pp. 186-94. doi : 10.1196 / annals.1299.032 . PMID 15033718 .
- ↑ J. Li, B. Lee, AS Lee: Endoplasmic reticulum stress-induced apoptosis: multiple pathways and activation of p53-up-regulated modulator of apoptosis (PUMA) and NOXA by p53 . (PDF) In: J. Biol. Chem. . 281, No. 11, March 2006, pp. 7260-7270. doi : 10.1074 / jbc.M509868200 . PMID 16407291 .
- ↑ H. Shiraishi, H. Okamoto, A. Yoshimura, H. Yoshida: ER stress-induced apoptosis and caspase-12 activation occurs downstream of mitochondrial apoptosis involving Apaf-1 . In: J. Cell. Sci. . 119, No. Pt 19, October 2006, pp. 3958-66. doi : 10.1242 / jcs.03160 . PMID 16954146 .
- ↑ CG Zou, XZ Cao, Zhao YS et al .: The molecular mechanism of endoplasmic reticulum stress-induced apoptosis in PC-12 neuronal cells: the protective effect of insulin-like growth factor I . In: Endocrinology . 150, No. 1, January 2009, pp. 277-285. doi : 10.1210 / en.2008-0794 . PMID 18801901 .
- ↑ Luis E. Muñoz, Christoph Peter, Martin Herrmann, Sebastian Wesselborg, Kirsten Lauber: Scent of dying cells: . The role of attraction signals in the clearance of apoptotic cells and its immunological consequences. In: Autoimmunity Reviews . 2009, doi : 10.1016 / j.autrev.2009.11.016 .
- ↑ K. Lauber, SG Blumenthal, M. Waibel, S. Wesselborg: Clearance of apoptotic cells; getting rid of the corpses . In: Molecular Cell . tape 14 , no. 3 , 2004, p. 277–287 ( sciencedirect.com [accessed August 28, 2013]).
- ↑ U. S, Gaipl, LE Munoz, G. Grossmayer, K. Lauber, S. Franz, K. Sarter, RE Voll, T. Winkler, A. Kuhn J. Kalden et al .: Clearance deficiency and systemic lupus erythematosus ( SLE). In: J Autoimmune . tape 28 , 2007, pp. 114–121 , doi : 10.1016 / y.jaut.2007.02.005 .
- ↑ J. Savill, N. Hogg, Y. Ren, C. Haslett: Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis . In: The Journal of Clinical Investigation . tape 90 , no. October 4 , 1992, doi : 10.1172 / JCI116019 .
- ^ G. Wickman, L. Julian, MF Olson: How apoptotic cells aid in the removal of their own cold dead bodies. In: Cell Death and Differentiation. Volume 19, 2012, 735-742, doi: 10.1038 / cdd.2012.25 .
- ^ LE Muñoz, K. Lauber, M. Schiller, AA Manfredi, M. Herrmann: The role of defective clearance of apoptotic cells in systemic autoimmunity. In: Nat Rev Rheumatol . Volume 6, No. 5, 2010, pp. 280–289, doi: 10.1038 / nrrheum.2010.46 .