Nucleosynthesis
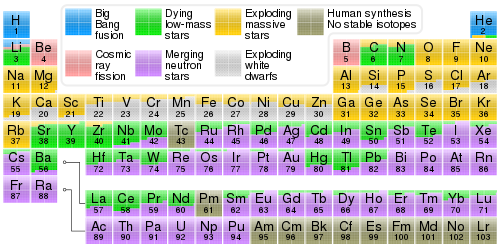
( link for exact percentages by swiping over the mouse )
The Nukleosynthese (from latin nucleus , nuclear ', nucleus ' and ancient Greek synthesis ', Bundling' Structure - also as Nukleogenese or element formation hereinafter) is the formation of nuclei and thus the chemical elements . A distinction is made between the
- primordial nucleosynthesis shortly after the big bang
- and stellar nucleosynthesis, which is mainly based on nuclear fusion , as well as r- , s- and p-processes .
Primordial nucleosynthesis began when the temperature in the universe had dropped so far that deuterium was no longer destroyed by high-energy photons . It ended about three minutes after the Big Bang.
The stellar nucleosynthesis takes place inside all stars . In the course of the evolution of a star there are characteristic nuclear fusions; initially helium is formed , later heavier elements up to iron , whereby energy is released which the star emits as radiation (which "makes it a star").
In contrast, energy is required for nuclear fusion to form elements with a higher atomic number than iron. They do not arise during the stellar nucleosynthesis, but at the end of the star's life when it explodes into a supernova ; but this only happens with stars that are big enough for it. The heavy elements are generated by proton and neutron capture reactions in p, r and s processes.
Elements on earth down to iron (see PSE ) can have arisen in the course of the life of our sun predecessor; all elements on earth with higher atomic numbers than iron come from its supernova explosion. Even heavier, always radioactive elements are created artificially in nuclear reactors and in targeted experiments.
Place of origin of chemical elements: Big Bang and stars
The nuclei of chemical elements heavier than hydrogen are continuously created by nuclear reactions inside stars. Even before the first stars could form, deuterium , helium-3 , helium-4 and traces of lithium -7 were already formed in the course of primordial nucleosynthesis . This nucleosynthesis is the subject of research in nuclear astrophysics and plays an important role in the field of cosmochemistry .
In stellar nucleosynthesis, a distinction is made between two subgroups of chemical elements with regard to their origins:
- The first group comprises nuclides , which are built up from the light starting materials by mostly exothermic fusion reactions between charged particles inside stars. The stars get their energy from these reactions, since the binding energy of an atomic nucleus per nucleon reaches its absolute maximum in iron and nickel (see figure). The proton-proton reaction and the Bethe-Weizsäcker cycle create helium-4 nuclei in the stars. The three-alpha process creates carbon- 12 nuclei in more massive stars .
- The exothermic fusion process ends with the elements nickel and iron. The isotope nickel-62 achieves the highest binding energy . Some endothermic processes also take place inside stars and can create even heavier nuclei. Far more iron-56 is produced than nickel-62, and the real reason for this and the end of the fusion chain lies in the details of the fusion process and the strong influence of photo-disintegration in this area.
- The second group includes the elements heavier than iron. Their formation ( synthesis ) by nuclear fusion requires an energy supply. The necessary energy comes from star explosions ( novae , supernovae, etc.), the merging of neutron stars and radioactive processes in AGB stars .
Big Bang, star formation and evolution

Within the first three minutes after the Big Bang , hydrogen ( nuclei ) and helium (nuclei) were formed at high temperatures and densities (see primordial nucleosynthesis ). The first stars formed from the hydrogen and helium gas clouds through the forces of attraction. In these stars, heavier elements were created through fusion processes.
Synthesis of light nuclides in young stars
The hydrogen supply of the sun and that of other stars is exhausted over time. When a star has "burned" most of the available hydrogen into helium in its central region, this first burning phase ends. The star can then no longer maintain its internal pressure and collapses under the influence of its own gravity . From a certain minimum mass, the compression and simultaneous heating create conditions under which further fusion processes start, initially the so-called helium burning . Depending on the initial mass, further fusion processes set in, see star (last burning phases) .
Thermonuclear fusion reactions are very dependent on the temperature inside the star. Therefore, the mass of the star determines to what extent the heavier elements can be burned in the course of the star's life. Lighter stars often do not get beyond the burning of helium due to the lower pressure inside, stars like our sun mainly produce the lighter elements up to carbon, while stars that are significantly heavier than the sun can produce all elements up to iron . This is where the positive energy balance of the fusion reactions ends. The inner core of such giant stars then consists of iron, followed by the other elements in layers outwards, a hydrogen-helium mixture forms the outermost layer.
In the 1940s, Fred Hoyle recognized that stars last had an onion skin pattern in their structure . His calculations showed that stars become increasingly inconsistent in their structure with the progressive depletion of their nuclear fuel and that this again causes higher temperatures and densities in their interior. The model agrees surprisingly well with the measured element abundances in the universe . How often the cycle of contraction, heating and ignition of new, heavier fuel is repeated depends only on the mass of the star. Star evolution drives nucleosynthesis, and at the same time nucleosynthesis drives star evolution again.
Synthesis of heavy nuclides
White dwarfs
For example, with the element iron, the fusion comes to a standstill. A fusion of iron into even heavier elements can no longer release any energy and is therefore not possible as a thermonuclear process. The star goes out and contracts under its own gravity. Its further fate depends on its original mass. With a mass on the order of our Sun or below, the star will repel part of its outer shell. It ends up as a faintly glowing white dwarf , which can take billions of years to cool down.
Synthesis of heavy nuclides in supernovae

If the star initially had a mass of more than 8 solar masses, the contraction progresses particularly quickly and the star collapses. With this rapid compression, the gravitational energy is released very quickly, increases the temperature sharply and thus causes an explosive expansion of the possible nuclear reactions in the entire star volume. Within one to two days the brightness of the previously inconspicuous star increases so much that, as described by Tycho Brahe in 1572 (see SN 1572 ), it appears brighter than all planets and can even be observed with the naked eye during the day: a supernova . This burst of luminosity lasts a few days. The outer part of star matter, sometimes more than half the total mass, is hurled into interstellar space.
The second group, the elements heavier than iron, is created in this explosive cloud of matter. These reactions are primarily caused by neutrons , which are released under the conditions prevailing inside the star and, as uncharged particles, can trigger a variety of nuclear reactions. Atomic nuclei capture a number of neutrons in rapid succession ( r-process ). In subsequent beta decays , stable nuclides with an increased number of protons, the heavy elements beyond iron, are formed from the neutron-rich nuclei .
The turbulent processes in a supernova not only ensure that the stars release the elements formed in them into space, they also create a whole new group of heavy chemical elements. Supernovae are thus the engines of an ongoing transmutation process ; their scattering material forms the starting material for the next generation of stars and planets . Therefore, as the universe ages, the amount of heavy elements increases. The supernova SN 2006gy in the galaxy NGC 1260 had 150 solar masses and when it exploded it released an estimated 20 solar masses into the universe in nickel alone .
In supernovae, the light elements lithium , beryllium and boron , which were "ignored" in the fusion reactions in the young star, are also formed through spallation (breaking up of atomic nuclei) .
Formation of the individual chemical element groups
Astro- and cosmochemistry paint the following picture of the exact origin and distribution of the individual chemical elements in the universe. Around 13.8 billion years ago the universe began to expand from a single point (Big Bang), whereby it initially had unimaginable amounts and density of energy (temperature around 10 32 Kelvin ). Before there was even a single atom of any element, just 10 −32 seconds after the Big Bang, the universe cooled down to about 10 28 Kelvin. Under these conditions, the first elementary particles could arise in the hot “energy pulp” of the young universe: the quarks , gluons and leptons .
The universe continued to cool - to the point that the quarks previously present as plasma condensed into protons and neutrons, the nucleons . This happened about 10 −7 seconds after the Big Bang at 10 14 Kelvin. But antineutron (n *) and antiproton (p - ) also arose . Since then, particles of matter and antimatter particles have been mutually destroying each other, converting them into energy . Example:
- p + + p - → photons (= energy)
This process can also run in the opposite direction ( pair formation ), but in the expanding universe the temperature decreased so that the process no longer takes place thermally. However, when the universe had reached a temperature of less than 10 14 Kelvin and all antimatter particles had annihilated themselves with matter particles, only a "tiny" residue, a "small excess" of matter remained (presumably by a mechanism similar to the CP violation ) . The most stable and most common representatives of this normal matter are protons, neutrons and electrons.
First fusion processes after the Big Bang
The primordial nucleosynthesis is the first action after the Big Bang. About 10 −2 seconds after the Big Bang, nuclei of heavy hydrogen ( deuterium , D) and helium isotopes (He) were created from the free-flying nucleons .
Only the atomic nuclei of hydrogen ( 1 H and 2 D) and helium ( 3 He and 4 He) as well as traces of lithium ( 7 Li) were formed during this primordial nucleosynthesis - in a ratio of 25 percent helium-4 and 75 percent hydrogen. The heavier elements that can be observed today come from fusion reactions in stars and thus from much later times. The first fusion of hydrogen to helium thus happened long before the first fixed stars could form from the hydrogen gas: The primordial nucleosynthesis only lasted about three minutes and took place simultaneously everywhere in the entire universe. The temperature at this point in time was still 10 10 Kelvin. After that, the temperature and density of the universe fell below the values required for nuclear fusion .
Five minutes after the Big Bang, the particle density of the universe fell so low that primordial nucleosynthesis ended. The remaining free neutrons decayed over the next few minutes.
When the temperature dropped below the corresponding binding energy (E> k B T) of the shell electrons, the atomic nuclei combined with electrons to form the first atoms
- p + + e - → H atom (hydrogen).
The age of atomic matter began with the chemical element hydrogen. The fact that the abundance of lithium in the atmospheres of early stars is two to three times less than current models of cosmological nucleosynthesis predict (which have proven to be reliable in terms of the abundance ratio of hydrogen to helium) is known as the primordial lithium problem .
First stellar nuclear fusion: hydrogen fuses to form helium
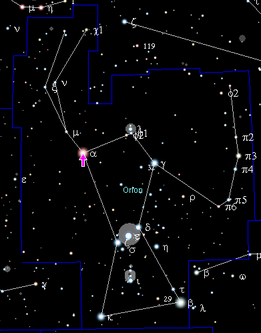
The universe has been expanding and cooling since the Big Bang. It took 10 13 seconds (300,000 years) until the gas mixture of hydrogen (H) and a few percent helium (He) was able to contract into dense clouds due to the effect of gravity . This was accompanied by such a strong increase in temperature that the necessary activation energy for further fusion processes was finally available in their centers. Stars lit up, like in the Orion Nebula , and in the so-called stellar hydrogen burn, the atomic nuclei of hydrogen melted into helium - the temperature required for this is around 10 million Kelvin.
If Deuterium D is involved, the corresponding process is also called " Deuterium burning ".
Reactions (selection)
D + D → T + p + 4.03 MeV D + T → 4 He + n + 17.588 MeV (largest cross section) D + D → 3 He + n + 3.268 MeV D + 3 He → 4 He + p + 18.34 MeV
In the sun, too, fusion reactions with the product 4 He take place with the release of energy , in the form of the proton-proton reaction . In addition, a carbon-catalyzed fusion cycle takes place in the sun, the CNO or Bethe-Weizsäcker cycle , which makes up around 1.6 percent of the energy in the solar household. Stars with less than 0.08 solar masses never reach the hydrogen fusion stage - they are called brown dwarfs .
The ash of both forms of hydrogen burning is Helium 4 He. When our sun's hydrogen supply is burned out in around 5 billion years, its core will only consist of helium. It will inflate itself so much that it swallows the inner planets Mercury and Venus that its disk in the earthly sky will be over 100 times larger than today.
Helium burning
One example is Betelgeuse in Orion, an inflated giant star (spectral class M2, 700 to 1000 times the solar diameter), it consists almost entirely of helium and has hardly any hydrogen reserves. At the end of the life of a star, when the hydrogen is used up, a star inflates and a new nuclear reaction starts in the now even more compressed center: the burning of helium. Additional energy now comes from the fusion of helium to carbon and oxygen (through the three-alpha process ). First generation stars initially only contained lighter elements - isotopes of carbon, oxygen, and heavier elements were only found in stars of later generations. The fusion of helium to "metals" such as carbon, oxygen and - later - also to silicon provides less energy than hydrogen burning. It requires higher pressures and temperatures than hydrogen fusion.
In astronomy , unlike in chemistry, any chemical element with an atomic number higher than helium is called "metal", and metallicity indicates how high the content of a star is in elements that are heavier than helium. Only hydrogen and helium are - together with some traces of lithium - the only elements that are present in the universe after the Big Bang. All other elements come from former stars in which they were generated by nuclear fusion , or from supernova explosions. The metallicity of objects in space can therefore also be understood as an indicator of its stellar activity.
Origin of the "metals"
Heavier stars can build up a higher gravitational pressure, which enables the fusion of heavier elements up to a mass number of 60. In the center of stars from 0.4 solar masses, the nuclear reaction from helium to carbon is initially possible after the hydrogen burn. From 0.7 solar masses, carbon fusion becomes possible, in which two carbon atoms each fuse to form neon, helium or sodium and protons, as well as magnesium and protons or neutrons. After hydrogen and helium, the elements carbon, neon, sodium and magnesium are the next most common basic substances in the universe, followed by the elements oxygen, silicon, phosphorus and sulfur.
Oxygen is also produced in the course of burning helium. From around 1.4 billion Kelvin, two oxygen atomic nuclei each fuse together (releasing helium, hydrogen, protons and neutrons) to form silicon-28, phosphorus-31 or the two sulfur isotopes sulfur-31 and -32, possibly also to chlorine and Argon.
Betelgeuse , the red shoulder star in the constellation Orion, is probably just as much a star as Antares , the deep red shining main star in Scorpio. Both belong to the red giant category , have consumed almost all of the hydrogen and started burning helium. Such a star is sooty: carbon is formed in it, and soot is also released from it by the star wind.
Stars with more than 10 solar masses reach central temperatures in which the formation of elements up to iron is possible, and the faster the more massive they were when they were formed. A star with 20 solar masses ultimately hurls several solar masses of matter into space when it explodes as a supernova . From the scraps of such a supernova explosion, our sun must once have formed as a third or fourth generation star - cosmochemistry tries to reconstruct the formation of the solar system on the basis of the frequency distribution of the isotopes from that supernova explosion. At temperatures of over 4 billion Kelvin, elements that are even heavier than iron were produced here , with heavy atomic nuclei, for example, merging to form uranium atoms while absorbing energy from the explosion : In every atomic bomb explosion and in every nuclear power plant, we can only extract the energies from the fuel elements that the explosion of supernovae was burned into those super-heavy atomic nuclei - the thermonuclear primal fire from which our solar system arose.
The carbon burning
The burning of carbon is a nuclear fusion reaction following the burning of helium, through which energy and heavier elements are generated in massive stars with an initial mass of at least 4 solar masses through the fusion of carbon . It occurs after the fusion of lighter elements has stalled. It requires high temperatures of over 6 · 10 8 Kelvin and densities of over 2 · 10 8 kg / m³. When burning carbon, two carbon nuclei 12 C are converted into other nuclei in a series of reactions - this is how the elements 24 Mg (also the isotope 23 Mg), 23 Na , 20 Ne and 16 O are formed
The carbon burning does not begin until the helium burning has come to a standstill. During the burning of helium, the now red, bloated giant stars convert helium (He) into carbon and oxygen faster and faster, until there is no longer enough helium to maintain the fusion: collapse begins. The inactive core, consisting mainly of carbon and oxygen, then collapses due to the force of gravity , which causes an increase in temperature and density until the ignition temperature for the carbon burning is reached. The radiation pressure then generated stabilizes the core and its further contraction is temporarily stopped. Due to the increase in temperature inside the star, helium burning can start again in a shell around the core area, now known as the so-called shell burning .
Burning neon
During carbon burning, the core area is enriched with the reaction products oxygen, magnesium and neon (Ne), until after a few thousand years the carbon is used up and the core cools down and contracts again. This contraction causes the temperature to rise until the neon can start burning. The shell burning of carbon then sets in around the core of the star, and further outside of the star, helium and hydrogen.
Stars with masses between 4 and 8 solar masses now become unstable and repel their outer shells via a strong stellar wind , creating a planetary nebula . What remains is the core of the star as a white dwarf , consisting of oxygen, neon and magnesium. Stars with masses greater than 8 solar masses continue to burn neon and eventually fuse all the lighter elements down to iron. The individual firing phases merge faster and faster.
Oxygen burn
The oxygen burning affects star with an initial mass of at least eight solar masses . It sets in after the lighter elements have been transformed through other fusion processes. The prerequisites for oxygen burning are high temperatures of at least 1.5 · 10 9 Kelvin and high densities of at least 10 10 kg / m 3 .
When burning oxygen, two oxygen nuclei 16 O fuse to form different new nuclei, including sulfur (S), phosphorus (P), silicon (Si) and magnesium (Mg). In addition, gamma quanta , neutrons n, protons or hydrogen nuclei 1 H (proton) and alpha particles ( helium nuclei ) 4 He are released.
During the previous neon glow, an inactive core of oxygen and magnesium formed in the central area of the star. In the absence of further fuel, the neon burn comes to a standstill. The radiation pressure is no longer sufficient to counteract the gravitation of its own mass, and the core is further compressed. This causes a renewed increase in temperature and density until the ignition temperature for the oxygen burn is reached and the star stabilizes again. Around the core, the so-called shell burning starts again the neon burning ; on the outside follow shells with carbon, helium and hydrogen fusion processes.
The oxygen burn only lasts a few years. During this time the core becomes enriched with silicon until the oxygen is consumed. The core then cools down again and is compressed by gravity until the last burning stage starts, the silicon burning.
Silicon firing
The silicon-burning process requires the star center, very high temperatures of at least 2.7 x 10 9 Kelvin and an extremely high density of at least 3 x 10 10 kg / m 3 . Due to their large Coulomb repulsion, two 28 Si nuclei cannot react directly with one another. Instead, the nuclei generated during oxygen burning are destroyed by photo-disintegration of photons. The fragments accumulate alpha particles, protons or neutrons in a series of steps. As a result, the iron isotope 56 Fe is ultimately reached.
The silicon burn follows the oxygen burn, which ends when the oxygen runs out in the central area of the star. As at the end of the previous firing phases, the now silicon-rich core is further compressed by gravity due to the lack of radiation pressure . This increases the temperature and density until the requirements for silicon firing are reached. The star thus reaches a hydrostatic equilibrium between gravity and radiation pressure for the last time. While the silicon is burning in the core, the oxygen, neon, carbon, helium and hydrogen burning continues in shells around the core.
The silicon burning represents the end of thermonuclear burning processes constitute the supply of nuclear fuel inside is consumed in silicon burning depending on the mass of the star in a few hours to a few days, and the gravitational collapse follows the most powerful explosion known in the Universe. A supernova of Type II.
Formation of the heaviest elements in supernovae
On the other hand, elements with mass numbers greater than 60 can no longer be created by stellar firing processes. The fusion of the corresponding nuclei consumes energy ( endothermic ) instead of releasing it. Since elements with higher mass numbers exist, there must be further possibilities for nucleosynthesis. After the star has completely burned out, it now goes out for good. The stabilizing radiation pressure falls away and the core collapses. It contracts under the action of its own gravity.
- At a mass on the order of our Sun or less, the star will repel part of its outer shell. It ends up as a faintly glowing white dwarf that takes billions of years to cool down.
- With a mass of 8 solar masses or more, the contraction proceeds very quickly and the star implodes. During this compression, a large amount of gravitational energy is released, which causes a considerable increase in temperature and thus an explosion-like expansion of possible nuclear reactions in the entire star volume. Within a day or two, the previously inconspicuous star increases in brightness so enormously that, as described by Tycho Brahe in 1572, it appears brighter than all planets and can even be observed with the naked eye during the day. This huge burst of luminosity only lasts a few days. A supernova has arisen in which the outer part of star matter, sometimes more than half its total mass, is thrown into interstellar space.
In this explosive cloud of matter, the second group of elements is created, which are heavier than iron. Rather, they are formed by neutrons ( s and r processes ) and proton accumulation ( p process ). Mainly involved in these reactions are the neutrons , which are released inside the bursting star under the extreme conditions prevailing there and, as uncharged particles, can trigger a variety of nuclear reactions. If atomic nuclei get into such a neutron flux, they capture a number of neutrons in rapid succession , similar to a reactor . In subsequent beta decays, stable isotopes with an increased number of protons develop from the neutron-rich nuclei , the last, heavy elements beyond iron.
The turbulent conditions in the matter clouds of the supernovae not only ensure that the stars release the elements formed in them into the vastness of the universe, but they also create a whole new group of heavy chemical elements. Supernovae at the end of the stellar nucleosynthesis are thus the engines of a creation process that will last into the distant future; their scattering material forms the starting material for the next generation of galaxies, stars and planets.
literature
- Margaret Burbidge , Geoffrey Ronald Burbidge , William Alfred Fowler , Fred Hoyle : Synthesis of the Elements in Stars . In: Reviews of Modern Physics . tape 29 , no. 4 , 1957, pp. 547-650 , doi : 10.1103 / RevModPhys.29.547 . The work is also known as B²FH .
- Claus E. Rolfs , William S. Rodney: Cauldrons in the Cosmos: Nuclear Astrophysics (Theoretical Astrophysics Series) . Univ. of Chicago Pr., Chicago 1988, ISBN 0-226-72456-5 .
- Heinz Oberhummer : Cores and Stars: Introduction to Nuclear Astrophysics . Barth, Leipzig / Berlin / Heidelberg 1993, ISBN 3-335-00319-5 .
- Vanessa Hill : From lithium to uranium - elemental tracers of the early chemical evolution. Cambridge Univ. Press, Cambridge 2005, ISBN 0-521-85199-8 .
- Andrew McWilliam, Michael Rauch: Origin and evolution of the elements. Cambridge Univ. Pr., Cambridge 2004, ISBN 0-521-75578-6 .
- Bernard EJ Pagel: Nucleosynthesis and chemical evolution of galaxies. Cambridge Univ. Press, Cambridge 1997, ISBN 0-521-55958-8 .
Individual evidence
- ↑ MP Fewell: The atomic nuclide with the highest mean binding energy . In: American Journal of Physics . tape 63 , no. 7 , 1995, p. 653-658 , doi : 10.1119 / 1.17828 .
- ↑ E. Pian, P. D'Avanzo, S. Benetti, M. Branchesi, E. Brocato: Spectroscopic identification of r-process nucleosynthesis in a double neutron-star merger . In: Nature . tape 551 , no. 7678 , November 2017, ISSN 0028-0836 , p. 67–70 , doi : 10.1038 / nature24298 ( nature.com [accessed November 21, 2019]).
- ↑ Darach Watson, Camilla J. Hansen, Jonatan Selsing, Andreas Koch, Daniele B. Malesani: Identification of strontium in the merger of two neutron stars . In: Nature . tape 574 , no. 7779 , October 2019, ISSN 0028-0836 , p. 497-500 , doi : 10.1038 / s41586-019-1676-3 ( nature.com [accessed November 21, 2019]).
- ↑ Maria Lugaro, Falk Herwig, John C. Lattanzio, Roberto Gallino, Oscar Straniero: s ‐ Process Nucleosynthesis in Asymptotic Giant Branch Stars: A Test for Stellar Evolution . In: The Astrophysical Journal . tape 586 , no. 2 , April 2003, ISSN 0004-637X , p. 1305-1319 , doi : 10.1086 / 367887 ( iop.org [accessed November 21, 2019]).
- ↑ Bodansky, David and Clayton, Donald D. and Fowler, William A .: Nucleosynthesis During Silicon Burning . In: Physycal Review Letters . tape 20 , no. 4 , 1968, p. 161--164 , doi : 10.1103 / PhysRevLett.20.161 (English).
- ↑ Hannu Karttunen, Pekka Kröger, Heikki Oja, Markku Poutanen, Karl Johan Donner: Fundamental Astronomy . 5th edition. Springer, Berlin / Heidelberg / New York 2007, ISBN 978-3-540-34143-7 , 10.3 Stellar Energy Sources, p. 237 (English, Finnish: Tähtitieteen perusteet . Helsinki 2003.).