Oleochemistry
The oleochemicals (also fat chemistry ) is a branch of chemistry that deals with the study of plant and animal fats , derived products, as well as the petrochemical busy manufactured product equivalents. Since oleochemistry is mainly concerned with chemistry based on renewable raw materials , it is also closely linked to the concept of sustainability .
In chemistry, vegetable and animal fats are referred to as triacylglycerides (triglycerides) because they consist of a glycerine residue to which three fatty acid residues are bound via ester bonds . The fatty acid residues consist of saturated, mono- or polyunsaturated, unbranched or branched or otherwise modified carbon chains with about 10 to 20 or more carbon atoms, so that there is a great variety. Oleochemistry deals with these compounds and the substances derived from them and the corresponding chemical reactions and manufacturing processes.
history
Oils and fats are an important and high-energy component of human nutrition. But the material use is also historically significant. The manufacture of soaps can be seen as the first oleochemical application of oils and fats . The energetic use, for example as fuel for oil lamps , was also known early on .
Modern oleochemistry began in the 19th century and a systematic study of properties and chemical reactions was carried out. Nowadays, oleochemistry is represented in many areas of life such as food production , cosmetics , pharmaceuticals and for the production of basic industrial chemicals as well as in the energy sector in the production of the biofuel biodiesel . About a seventh of the globally produced amount of oils and fats is processed oleochemically.
Raw material base
The naturally occurring fats and oils differ mainly in the chain length distribution and the number of double bonds in the carbon chain.
| fatty acid | Chain length | Main source | Main application |
|---|---|---|---|
| Caprylic acid | 8th | Cuphea | cleaning supplies |
| Capric acid | 10 | Cuphea | cleaning supplies |
| Lauric acid | 12 | Coconut palm | cleaning supplies |
| Myristic acid | 14th | nutmeg | Soap |
| Palmitic acid | 16 | Oil palm | Margarine, biodiesel |
| Petroselinic acid | 18th | coriander | cleaning supplies |
| Oleic acid | 18th | Rapeseed | Edible oil, biodiesel |
| α-linolenic acid | 18th | Flax, camelina | linoleum |
| Calendulic acid | 18th | Marigold | Perfume, lubricant |
| Ricinoleic acid | 18th | Ricinus | lubricant |
| Vernolic acid | 18th | Vernonia , Euphorbia | Epoxy resins |
| α - eleostearic acid | 18th | Aleurites | paint |
| Eicosenoic acid | 20th | Limnanthes | lubricant |
| Erucic acid | 22nd | Rapeseed, Krambe | lubricant |
| Nervonic acid | 24 | Lunaria | lubricant |
In 2016, soybean oil was the most widely produced vegetable oil with a share of 61% worldwide, ahead of palm and rapeseed oil . The total consumption in 2016 was 340.8 million tons.
The following table shows the proportions of the various fatty acid residues in triglycerides of technically important oils in percent, with the number of carbon atoms and double bonds in the molecule in brackets .
| substance |
origin |
Capryls. (8) , caprins. (10) , Laurins. (12) |
Myristic acid (14) |
Palmitic acid (16) |
Stearic acid (18) |
Arachidic acid (20) |
Behenic acid (22) |
Oleic acid (18: 1) n-9 |
Linoleic acid (18: 2) n-6 |
Linolenic acid (18: 3α) n-3 |
Gamma- linolenic acid (18: 3γ) n-6 |
(20: 1.2) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Palm kernel oil | Palm fruit (kernels) | 57 | 16 | 8th | 2.5 | 14th | 2.5 | |||||
| Palm oil | Palm fruit (pulp) | 1 | 43.8 | 5 | 0.5 | 39 | 10 | |||||
| Rapeseed oil | Rapeseed (seeds) | 4th | 1.5 | 0.5 | 63 | 20th | 9 | 1 | ||||
| Soybean oil | Soybean | 10 | 4th | 23 | 51 | 7 (or <1) |
Applications

Renewable raw materials currently cover around ten percent of the raw material requirements of the chemical industry, a large part of which through oleochemicals. One of the largest applications in terms of quantity today is the production of biodiesel by transesterification with methanol .
The production of surfactants from the fatty acid lauric acid to sodium lauryl sulfate ( anionic surfactants ), an important ingredient in many skin care products, is a large-scale process. With sugar surfactants ( nonionic surfactants ) the fatty acid is bound to a sugar .
Other applications are the production of lubricating oils , solvents and bio- and copolymers for the plastics and paint sector . Glycerine is used in many ways in the cosmetics sector. Since it is available in large quantities through biodiesel production, it is also used as animal feed, energetically and otherwise.
Chemical processes
Oleochemistry mostly takes place on the carboxy group (-COOH) as the functional group of the fatty acids. So far, the chemistry of the fatty acid chain has been little used, with the exception of castor oil , whose fatty acid chain naturally has a hydroxyl group. Their research could represent a great technical potential and is supported by programs of the Federal Ministry of Food, Agriculture and Consumer Protection (BMELV).
The biogenic origin of the oils makes them predestined for biotechnological implementation, which has also been little researched to date.
Reactions on the carboxy group
hydrolysis
By hydrolysis of triglycerides is obtained fatty acids and glycerol :
The reaction can be carried out with either acid or base catalysis. The subsequent chemistry of fatty acids is diverse. It includes the production of nitriles and their secondary products, fatty acid amides and acid chlorides .
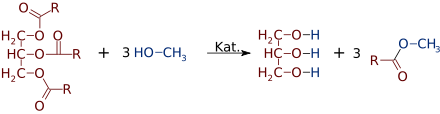
Transesterification
The reaction of fats and oils with an alcohol is called transesterification. The process is used on an industrial scale for the production of biodiesel. This reaction is also carried out with base catalysis in technology. When fully converted, a triacylglyceride is converted into three fatty acid methyl esters (FAME), which make up biodiesel, and a molecule of glycerine as a by- product .
The resulting methyl esters of unsaturated fatty acids can enter into a wide range of secondary reactions , such as hydrogenation , metathesis and epoxidation .
Saponification
The saponification is one of the oldest known chemical processes of humanity and has been from the Sumerians used. The triglyceride (= triester of glycerine) is converted with a base - here sodium hydroxide solution - to form the metal salt (here sodium salt ) of the fatty acid. The soap obtained in this way (= sodium salts of fatty acids) is one of the anionic surfactants . R 1 , R 2 and R 3 organyl residues ( alkyl or alkenyl residues) of fatty acids:
Hydrogenation
Hydrogenation of the methyl esters leads to fatty alcohols , which play an important role as raw material for the production of anionic surfactants. By ethoxylation and subsequent sulfation with gaseous sulfur trioxide leads to lauryl ether sulfates , which are used in the manufacture of personal care products.
Another application of fatty alcohols is the production of alkyl polyglycosides , a surfactant with a sugar residue (a sugar surfactant) as a hydrophilic group.
Reactions on the carbon chain
metathesis
By metathesis of unsaturated Fettsäureestern leads to a wide variety of secondary products, such as unsaturated dicarboxylic acid and internal olefins . Ethenolysis makes ω-unsaturated fatty acid esters accessible, with α-olefins as a by-product. These two products can in turn be used as components in the copolymerization component in the polymerization of ethylene using the Ziegler-Natta process and thus lead to functionalized polyethylene .
Epoxidation
Unsaturated methyl esters can be epoxidized by the Prileschajew reaction with an organic peroxycarboxylic acid and can be used as PVC stabilizers or as reactive solvents in paint chemistry.
Petrochemical processes
Oleochemistry also deals with the production of petrochemical-based oleochemicals. An example of this is the production of glycerine from propylene via the stages of allyl chloride , dichlorohydrin and epichlorohydrin , which can be converted into glycerine with sodium hydroxide solution .
See also
literature
- Siegfried Warwel, Nikolaus Weber: Lipids as functional foods. Landwirtschaftsverlag, Münster 2002, ISBN 3-7843-0495-8 .
Web links
Individual evidence
- ↑ Fatty acid composition of important vegetable and animal edible fats and oils .
- ↑ Challenges for the breeding of plants as renewable raw materials .
- ↑ International: World Oilseed Production. In: soystats.com. American Soybean Association, accessed November 6, 2017 .
- ↑ Deutsche Molasse Handelsgesellschaft mbH (DMH): What is glycerine? ( Memento of the original from February 2, 2010 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. Information page, accessed February 11, 2010.
- ↑ Renewable raw materials funding program ( Memento of the original from August 18, 2010 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. (Program of the Federal Ministry for Food, Agriculture and Consumer Protection).
- ^ Siegfried Warwel: Metathesis in oleochemistry (PDF; 161 kB).
- ↑ Mark Rüsch gen. Klaas: Epoxidized fatty acids and triglycerides. ( Memento from January 16, 2014 in the Internet Archive ) In: The current newsreel of the GDCh. 2008 (version from the Internet archive from January 16, 2014).
- ^ Karlheinz Hill, Manfred Weuthen: Alkylglucoside - surfactants from sugar and vegetable oil. In: Spectrum of Science. No. 6, 1994.
- ^ PB van Dam, M. C. Mittelmeijer, C. Boelhouwer: Metathesis of unsaturated fatty acid esters by a homogeneous tungsten hexachloride-tetramethyltin catalyst. In: Journal of the Chemical Society, Chemical Communications. No. 22, 1972, pp. 1221-1222. doi: 10.1039 / C39720001221 .