History of electrolysis
The history of electrolysis describes the most important steps that led to the discovery of electrolysis and the subsequent advances in its technical application.
Discovery of electrolysis, new syntheses, compounds and elements, first theories

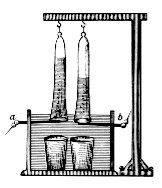
The first usable and efficient battery, the voltaic column , was completed by the Italian physicist Alessandro Volta in 1799. It was the very first voltage source that could cause a longer current flow. In 1800, Volta reported his discovery to the President of the Royal Society , Joseph Banks. In the same year the first electrolyses were carried out: William Cruickshank decomposed salt water. William Nicholson and Anthony Carlisle showed that water with electricity from the voltaic column produces two gases in a ratio of 2: 1. Johann Wilhelm Ritter was able to use the oxyhydrogen gas test and white phosphorus to prove that the two gases were hydrogen and oxygen.
As early as 1799, Ritter put together different tension chains with two metal sheets each, so that he could get a first tension series from base to noble metals. Ritter also recognized that chemical reactions take place in galvanic power generation. Humphry Davy investigated the formation of acids and bases using litmus paper and was thus able to study the migration of ions in the course of electrolysis.
Theodor Grotthuss (1785–1822) gave the first theory of water decomposition in 1805. He assumed that the tension created positive hydrogen particles and negative oxygen particles. According to this theory, however, the influence of salts, acids and bases on electrolytic deposition remained unclear. In 1802 Hisinger and Jöns Jakob Berzelius obtained chlorate electrochemically from table salt , although they have not yet been able to conclusively substantiate the result. But in 1847 Kolbe confirmed the formation of chlorate from hypochlorous acid. Hisinger and Berzelius hypothesized that molecules (atoms) have a positive and a negative pole (electropositive, electronegative) - similar to a magnet.
Humphry Davy developed an experimental setup in which the positive and negative poles were in two separate vessels. He was able to show that a sodium sulfate solution forms a weak sodium hydroxide solution at the negative pole and weak sulfuric acid at the positive pole. He then investigated the electrolysis of molten salts from 1805 to 1807, using a platinum spoon as the cathode and a platinum wire as the positive electrode. In 1807 he discovered potassium during the electrolysis of molten caustic potash, i.e. potassium hydroxide. A little later he received metallic sodium from caustic soda. These metals are easy to catch on fire or to react with oxygen in the air, and accordingly this discovery has generated considerable excitement and interest. In 1808 Davy produced metallic magnesium, calcium, strontium and barium.
In the novel Metall by Karl Aloys Schenzinger , the historical personalities of electrochemistry were presented in an interesting way.
Research into the fundamental laws
Michael Faraday studied electrolysis more closely from 1832. By weighing the amount of substance deposited ( coulometer ) after a certain time unit, he was able to determine the amount of electricity (amount of charge, coulomb A * s) at constant voltage. The Faraday constant was later named after him, which relates the amount of charge and the amount of substance deposited: to deposit 1 mol of substance (e.g. silver), an amount of charge of around 96485 coulombs is necessary. If the ion to be deposited is charged several times, the multiple charge of the ion with an integral multiple of the corresponding charge must be taken into account. These dependencies of the converted masses on the amount of charge and the molar mass are called Faraday's laws today . Faraday also compiled a table on cations and anions. Faraday also created a number of terms necessary to describe the results, taking advice from a Cambridge philosopher and mathematician, William Whewell. So they coined the new terms electrolysis , electrode , electrolyte , anode , cathode , anion and cation , published in Faraday's work 1832/1834.
In 1835, John Frederic Daniell dipped a copper sheet in a copper sulfate solution and a zinc sheet in a zinc sulfate solution. He separated both half-cells by a diaphragm (clay diaphragm). The Daniell element became the most important source of electrical energy for several years. In 1843 Robert Bunsen manufactured a very inexpensive battery with a zinc plate and a special carbon in dilute sulfuric acid. Until Werner von Siemens invented the dynamo, it was the best source of electricity.
Charles Wheatstone developed the bridge circuit to determine resistances, Johann Christian Poggendorff invented the Poggendorff compensation circuit to determine the voltages, Friedrich Kohlrausch developed a method for measuring the resistance of an electrolyte solution without polarizing the electrodes by means of alternating current, the Kohlrausch's square root law , so that the conductivities of electrolyte solutions are now examined could.
Johann Wilhelm Hittorf recognized the different migration speeds of the ions during electrolysis, and he introduced the transfer number . Friedrich Kohlrausch examined the conductivities of concentrated to very dilute salt solutions and was able to determine a linear relationship by graphically plotting the conductance values and the root of the salt concentration. In high dilution, every ion has a very characteristic conductance. Kohlrausch introduced molar conductivity .
Svante Arrhenius and Wilhelm Ostwald investigated the conductivity of weak acids, bases and salts in aqueous solutions. Based on the findings of Jacobus Henricus van 't Hoff that the osmotic pressure and the lowering of the freezing point of a liquid are proportional to the number of individual ionic particles in a solution, Arrhenius and Ostwald discovered the principle of dissociation of weak acids through conductivity measurements. Depending on the strength of the acid, only part of the acid is in ionic form. Ostwald (1881) derived a law ( Ostwald's law of dilution ) for calculating conductivities as a function of concentration and acid strength.
In 1923 Petrus Debye , Erich Hückel and Lars Onsager calculated the interaction of the ions with the dielectric constant of the solution and were able to carry out an even more precise mathematical theory of conductivity determination. The knowledge of the dissociation, the ionic conductivity in aqueous solutions, was of great importance for the pH determination, the conversion control of electrolyses, the determination of the salt content of solutions of unknown concentration.
Max Julius Le Blanc determined the voltage values (decomposition voltage) for the separation of substances from normal solutions, found a method for determining the individual electrode potentials and also introduced an oscilloscopic current-time measurement for electrolysis.
On the basis of electrochemical work, Hermann von Helmholtz coined the terms energy, free enthalpy (reaction driving force, heat development and state of order after a chemical reaction) and the dependence of an equilibrium on temperature.
Walther Nernst examined the electrolyte concentrations during oxidation and reduction processes and found an understandable explanation for metallic deposits on the cathode and the dissolution of anode metals. On the basis of the electrolyte concentration using the Nernst equation , deposition voltages and also electrochemical redox equilibria can be determined.
Nernst gave the half-cell of a platinum electrode surrounded by hydrogen in 1-molar hydrochloric acid, which is in equilibrium with the hydrogen formation, the arbitrary standard normal potential 0 V. On this reference point, all voltage values of other redox equilibria could now be determined and a uniform electrochemical voltage series could be established. Besides Cottrell, Nernst was also a pioneer in explaining diffusion processes .
Julius Tafel dealt with overvoltage (electrochemistry) on electrodes and developed a mathematical formulation for them.
Oliver Wolcott Gibbs and Alexander Classen developed the basics for the qualitative and quantitative deposition of metal ions in aqueous solutions using electrogravimetry .
Technical uses and advances in theory in the second half of the 19th century
In 1851, C. Watt received an English patent for a cell for the electrolytic production of chlorate from saline solution, which already had some of the characteristics of today's cells. However, since he did not yet have a dynamo available, it was not yet used; Chlorate was only produced electrosynthetically after 1886. After the invention of powerful electric generators - which, incidentally, were also based on the work of Michael Faraday -, among others also in 1866 by Werner von Siemens, who used the dynamo-electric principle, electrical currents became cheaper. In the years before the turn of the century there was a rapid development of technical electrolysis: From 1870 copper was obtained electrolytically in Germany, France and England. In 1875, Emil Wohlwill made improvements to copper, silver and gold refining in the North German affineries. Important locations for copper production in Germany were Mansfeld, Oker and the Norddeutsche Affinerie (today Aurubis AG) in Hamburg. Copper refining began in the United States in 1892. In 1910, US electrolytic copper production was over 400,000 tons.
In 1890, Hamilton Castner introduced a cell named after him for the electrolysis of molten sodium hydroxide. Here, the cylindrical cathode, which is located in the middle of an anode made of nickel tube, is separated from the anode by an iron wire mesh cylinder. A collecting bell picks up the resulting sodium. In 1892, Hamilton Castner in the USA and Karl Kellner in Austria invented the amalgam process for chlor-alkali electrolysis . This takes advantage of the fact that sodium dissolves as an amalgam in a mercury cathode, while the formation of hydrogen at the cathode is prevented by the high overvoltage. In 1890, the electron chemical factory in Griesheim, under the direction of Ignatz Stroof, built the first plant for chlor-alkali electrolysis using the diaphragm process. As early as 1908, 50,000 tons of sodium hydroxide could be produced using this process.
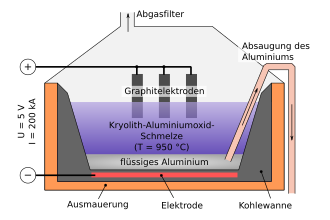
The first work on the electrowinning of aluminum was done by Robert Bunsen and St. Claire-Deville. In 1886, Paul Louis Toussaint Héroult and Charles Martin Hall from Ohio developed an electrolysis method for the production of aluminum, which is now called the Hall-Héroult process after the discoverers and is the basis of today's processes. Just two years later, companies were founded using this, and in 1900 80,000 tons of aluminum had already been electrolytically extracted. The process uses molten cryolite, sodium hexafluoroaluminate, in which aluminum oxide is dissolved, as the electrolyte. The electrolysis is carried out at approx. 950 ° C, with the aluminum collecting at the bottom of the electrolysis cell.
Boom in the first half of the 20th century
Production with the help of electrolysis processes showed strong growth in many cases until the downturn during the Second World War . For example, over 600,000 tons of chlorine were produced in the United States in 1940. Also hydrogen peroxide was then produced synthetically electro, with sulfuric acid was electrolyzed. There arise peroxosulfuric acid and peroxodisulfuric acid or salts thereof, which can be hydrolyzed with water to form hydrogen peroxide. From 1945 this process was replaced by chemical manufacturing methods.
The development of potentiostats , which began in the 1940s , made it possible to investigate electrochemical investigations including electrolysis under better controlled conditions, since the processes at the counter electrode do not influence the potential measurement, stimulated the following research into electrode processes.
Improvements and new syntheses in the second half of the 20th century
In 1968, Beer patented electrodes made of coated titanium , for example with ruthenium dioxide. These so-called “dimensionally stable anodes” quickly became the material of choice for the electrolytic production of chlorine, as they show less wear than graphite , the surface of which is gradually oxidized and thus eroded. At the end of the 1960s, DuPont brought ion exchange membranes made of fully fluorinated polymer to the market under the trade name “Nafion”, which combined excellent chemical stability with useful conductivity. This enabled the development of membrane processes for electrolysis. Electrolysis, with the aim of producing chlorate, experienced a considerable boom in the last decades of the 20th century, which was based on the increased demand for chlorine dioxide as a fiber-friendly and effective bleaching agent in the paper industry. Since chlorine dioxide cannot be transported, chlorate and chlorine dioxide are usually produced on site.
The strong demand for nylon mounted in the mid-sixties, the company Monsanto to an electrolytic process for the production of a precursor of the required for nylon production hexanediamine to develop: The electrolytic synthesis of adiponitrile (Hexandinitril) of acrylonitrile (propenenitrile). The acrylonitrile is cathodically reduced and dimerized with protonation. The desired hexanediamine is then obtained by catalytic hydrogenation .
Latest developments
Electrolyses of aqueous solutions in which no hydrogen is produced at the cathode, but instead oxygen is converted, require significantly lower voltages and can therefore save energy. Since the oxygen has to be brought to the cathode in sufficient quantities, special electrodes are required, so-called gas diffusion electrodes. At the end of 2003, Bayer AG commissioned a hydrochloric acid electrolysis system with oxygen-consuming cathodes in Brunsbüttel, which can produce 20,000 tons of chlorine per year.
Date list
- 1791 - Luigi Galvani reports "About the forces of electricity in muscle movement"
- 1791 - Alessandro Volta begins his work on electricity
- 1799 - Alessandro Volta completes his voltaic column , the first powerful battery
- 1800 - Volta reports to Sir Joseph Banks in a letter written in French entitled “On the electricity that is excited by the mere contact of various kinds of conductive substances”
- 1800 - William Cruickshank, Johann Wilhelm Ritter , William Nicholson and Anthony Carlisle carry out the first electrolysis.
- 1802 - Hisinger and Jöns Jakob Berzelius produce chlorate from table salt using an electrochemical method
- 1802 - William Cruickshank improves the voltaic column, also through series production
- 1807 - Humphry Davy represents sodium and potassium ago
- 1808 - Humphry Davy receives magnesium , calcium , strontium and barium
- 1818 - Humphry Davy obtained lithium metal through the electrolysis of lithium carbonate
- 1848 - Hermann Kolbe publishes his results on the electrolysis of valeric acid (pentanoic acid)
- 1849 - Hermann Kolbe reports on the electrolysis of other organic compounds. He represents z. B. Ethane from acetic acid ; the electrolysis of carboxylic acids to produce alkanes is called Kolbe electrolysis after him .
- 1851 - C. Watt receives an English patent for a cell for the electrolytic production of chlorate
- 1855 - Robert Wilhelm Bunsen receives large amounts of lithium metal through the electrolysis of a lithium chloride melt
- 1864 - The American chemist Oliver Wolcott Gibbs (1822–1908) uses electrolysis for quantitative analysis: He electrolytically deposits copper or nickel . From the change in mass of the electrode, the amount or concentration of the metal, e.g. B. copper determine. He thus founded electrogravimetry .
- 1867 - Werner von Siemens presents his dynamo, which enables the efficient provision of electrical energy
- 1884–1887 - Svante Arrhenius develops the theory of electrolytic dissociation
- 1885 - Breuer develops a diaphragm
- 1886 - Henri Moissan discovered by electrolysis of dissolved in anhydrous hydrofluoric acid , potassium fluoride , the fluorine
- 1886 - Charles Martin Hall from Ohio and Paul Héroult discover an electrolysis process for the production of aluminum, which is named after them the Hall-Héroult process .
- 1889 - Walther Nernst publishes the Nernst equation named after him in his habilitation , which describes the concentration dependence of the electrode potential
- 1890 - The first chlor-alkali electrolysis for the technical production of caustic soda is put into operation in Griesheim
- 1891 - Max Julius Le Blanc (1865–1943) works on the decomposition voltage of electrolytes
- 1890 - Hamilton Castner introduces a cell for the electrolysis of molten sodium hydroxide
- 1892 - Hamilton Castner and Karl Kellner independently register patents for a mercury cell for the production of sodium hydroxide
- 1902 - Max Julius Le Blanc produces chromium electrolytically
- 1968 - Henri Bernard Beer patents electrodes made of coated titanium, these improve the electrolysis for chlorine production
- 2003 - In Brunsbüttel, hydrochloric acid electrolysis is the first technical electrolysis with an oxygen-consuming cathode.
Individual evidence
- ↑ Handbook of Experimental Chemistry, secondary level II, Aulis Verlag Deubner & Co. KG, Cologne 1994, ISBN 3-7614-1630-X , p. 12 ff.
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 91st – 100th, improved and greatly expanded edition. Walter de Gruyter, Berlin 1985, ISBN 3-11-007511-3 .
- ↑ Wied. Annals 17 , 642 (1882).
- ↑ Poggendorfsche Annalen 89,98,103,106 (1853,1859).
- ↑ Wied. Annals 6 , 1 (1879), 26 , 213 (1885).
- ↑ Arrhenius, Zeitschrift fur Physical Chemistry, 1 , 631 (1887).
- ^ Journal of physical chemistry, 2 , 270 (1888).
- ↑ Journal for physical chemistry 8 , 299 (1891).
- ^ Journal of physical chemistry 12 , 333 (1893).
- ^ M. Le Blanc: Treatise of the Bunsen Society 3 (1910).
- ↑ Journal for physical chemistry 4 , 129 (1889).
- ^ Journal of physical chemistry 2 , 617 (1888).
- ↑ Journal for physical chemistry 47 , 52 (1907).
- ^ FG Cottrell, Journal of Physical Chemistry 42 , 385 (1903).
- ↑ H. Jahn, Journal for physical chemistry 26 , 408 (1898).
- ^ Journal of physical chemistry, 50 , 641 (1905).
- ^ W. Jansen: Handbook of experimental chemistry - secondary level II, Aulis Verlag Deubner & Co. KG, Cologne 1994, p. 26.
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 91st – 100th, improved and greatly expanded edition. Walter de Gruyter, Berlin 1985, ISBN 3-11-007511-3 , p. 932.
- ↑ Bolko Flintjer: Handbook of experimental chemistry - secondary level II, p. 308, Aulis Verlag Deubner & Co. KG, Cologne 1994.
- ^ Poggendorfs Ann. 92 , page 648 (1854).
- ^ A. Hickling: Studies in electrode polarization. Part IV.-The automatic control of the potential of a working electrode . In: Transactions of the Faraday Society . 38, 1942, pp. 27-33. doi : 10.1039 / TF9423800027 .
- ↑ J. Kintrup, Gas Diffusion Electrodes in Electrolysis ( Memento of the original from January 21, 2011 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice.