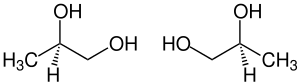
1,2 propanediol
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| ( R ) - (-) - shape (left) and ( S ) - (+) - shape (right) | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | 1,2 propanediol | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula | C 3 H 8 O 2 | |||||||||||||||||||||
| Brief description |
oily, colorless, almost odorless, hygroscopic, viscous liquid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 76.09 g · mol -1 | |||||||||||||||||||||
| Physical state |
liquid |
|||||||||||||||||||||
| density |
1.04 g cm −3 (25 ° C) |
|||||||||||||||||||||
| Melting point |
−60 ° C |
|||||||||||||||||||||
| boiling point |
188.2 ° C |
|||||||||||||||||||||
| Vapor pressure |
0.11 h Pa (20 ° C) |
|||||||||||||||||||||
| solubility |
miscible with water, ethanol , acetone , chloroform , soluble in diethyl ether |
|||||||||||||||||||||
| Refractive index |
1.4324 |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data |
|
|||||||||||||||||||||
| Thermodynamic properties | ||||||||||||||||||||||
| ΔH f 0 |
−501.0 kJ / mol |
|||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||||||||
1,2-propanediol ( 1,2-propylene glycol ), also known as propylene glycol , is a clear, colorless, almost odorless and highly hygroscopic liquid. 1,2-Propanediol is one of the polyvalent alkanols and is chiral at C2 , so there is an ( R ) enantiomer and an ( S ) enantiomer. Unless expressly stated otherwise, all information in this article relates to the 1: 1 mixture ( racemate ) of the ( R ) -enantiomer and the ( S ) -enantiomer.
Extraction and presentation
Industrial 1,2-propanediol by hydration of propylene oxide produced. Depending on the manufacturer, either a high-temperature process without catalysis at 200–220 ° C or a catalytic process at 150–180 ° C in the presence of an ion exchange resin or small amounts of sulfuric acid or alkalis is used. The end products of these processes contain 20% 1,2-propanediol (which is purified by rectification ), 1.5% dipropylene glycol, and minor amounts of other polypropylene glycols.
In 2007 the worldwide production capacity was 1,400,000 tons .
Enantiomerically pure ( S ) - (+) - 1,2-propanediol is accessible by the degradation reaction of D- mannitol.
properties
Propylene glycol is miscible with water and polar organic solvents. At high temperatures above 150 ° C it is sensitive to oxidation . It forms azeotropes with toluene and xylene , but not with water.
Due to its hygroscopic properties, aerosols of propylene glycol in the air act as condensation nuclei for mist droplets from the surrounding air humidity.
Safety-related parameters
1,2-Propanediol has a flash point of 101 ° C. The compound therefore only forms inflammable vapor-air mixtures above this temperature. The explosion range is between 2.6% by volume (80 g / m 3 ) as the lower explosion limit (LEL) and 12.6% by volume (400 g / m 3 ) as the upper explosion limit (UEL). The ignition temperature is 420 ° C. The substance therefore falls into temperature class T2.
Health risks
After decades of use, no serious health hazards have become known. Since 1942 is 1,2-propanediol in the USA an approved ingredient for medicines and by the Food and Drug Administration (FDA) as basically harmless ( generally recognized as safe ) approved for food and cosmetic applications. The acute and chronic or subchronic toxicity can be regarded as extremely low. The fatal oral dose for an adult is assumed to be 0.5 to 5 g per kg of body weight. The Joint FAO / WHO Expert Committee on Food Additives specifies a permitted daily dose of 25 mg per kg of body weight.
Indications of carcinogenic, mutagenic or reproductive toxicity properties could not be found. 1,2-Propanediol has no skin-irritating properties and only very minor eye-irritating properties.
According to a frequently cited study, occasional irritation of the eyes and throat was observed when inhaling mists made from 1,2-propanediol. The ECHA has been checking since March 2016 whether these effects are sufficient for labeling according to the CLP regulation for STOT SE 3, H335 . In December 2016, ECHA's Risk Assessment Committee (RAC) decided that these effects were not sufficient for classification.
The isolated occurrence of allergic reactions is considered certain. However, the trigger mechanism has not been clarified. The two enantiomers or possible impurities ( propylene oxide , 1,3-propanediol ) are possible triggers .
use
Because of its dissolving and emulsifying properties, 1,2-propanediol is used, among other things, as a carrier and carrier solvent for dyes , flavors , antioxidants , emulsifiers and enzymes .
In addition to water and glycerine (1,2,3 propanetriol), propanediol, as a component of fog fluids, provides the desired dense fog.
Food additive
1,2-Propanediol is approved in the EU as a food additive for chewing gum and dietary supplements in the form of capsules or tablets and has the designation E 1520 .
Propanediol is used as a carrier in the preparation of food flavors.
Cosmetics & hygiene
1,2-Propanediol is contained in cosmetic products such as skin creams, toothpaste, mouthwashes and deodorants as a humectant. The substance is used as a co- surfactant in multi-component systems and promotes the formation of water-in-oil emulsions . In addition, it can often contribute to a significant improvement in the absorption of various active ingredients. The antimicrobial effectiveness often makes the use of additional preservatives superfluous.
Heat transfer medium
Like ethylene glycol, 1,2-propanediol is suitable as a heat transfer medium in solar thermal systems . When used as a cooling liquid, the heat capacity varies from 2.5 to 4.2 kJ / (kg · K) for pure water, depending on the addition of water. A 50/50 mixture freezes at −35 ° C, boils at 104 ° C and reaches a heat capacity of 3.5 kJ / (kg · K). When used in refrigeration systems in food processing, only propanediol is permitted due to its non-toxicity.
Tobacco and electronic cigarettes
1,2-Propanediol is contained in almost all tobacco products as an additive , as it is added to both cigarette tobacco and water pipe tobacco together with glycerine as a humectant . High amounts of unburned humectants (glycerine and 1,2-propanediol) are found in the smoke from water pipes. The Federal Institute for Risk Assessment (BfR) warns of possible health hazards that could result from the absorption of these substances with tobacco smoke from water pipes and therefore recommends limiting the maximum amount of humectants to 5% as before. Propanediol is the main component (up to 75%, as of 2013) in liquids from electronic cigarettes . In addition, propanediol is used in an aqueous solution in the humidification systems of humidors ( relative humidity over 70%).
Use of propanediol in dairy cattle feeding
For some time now, 1,2-propanediol has also been used as a feed additive for dairy cows . Due to the constantly increasing milk yield required by the cows, which is now around 50 liters / day for high-performance dairy cows, the milk yield after calving is falling more and more frequently. Particularly in the so-called transit phase, the two weeks before calving, and in the first lactation phase, the additional administration of 1,2-propanediol to prevent ketosis and to stabilize performance has proven itself within the framework of suitable feeding strategies and feed rations .
Alternatively, 1,2,3-propanetriol (glycerine) can also be fed. As long as the qualitative regulations for feed are complied with, undistilled raw product quality from fatty acid production can also be used.
Use in medicine
In veterinary medicine, propanediol (usually in a 50% dilution) is used for excessive keratinization of the skin. It is also used as a carrier for pharmaceuticals (e.g. ivermectin , minoxidil ).
Individual evidence
- ↑ Entry on E 1520: Propane-1, 2-diol (propylene glycol) in the European database for food additives, accessed on June 27, 2020.
- ↑ Entry on PROPYLENE GLYCOL in the CosIng database of the EU Commission, accessed on December 23, 2019.
- ↑ Entry on propanediol. In: Römpp Online . Georg Thieme Verlag, accessed on September 29, 2014.
- ↑ a b c Maryadele J. ONeil; Merck & Co., Inc. (Ed.): The Merck Index : An Encyclopedia of Chemicals, Drugs, and Biologicals. 14 edition. Elsevier, Whitehouse Station, NJ, USA 2006, ISBN 978-0-911910-00-1 , p. 1350.
- ↑ a b c d e f g h i Entry on 1,2-propanediol in the GESTIS substance database of the IFA , accessed on December 21, 2019(JavaScript required) .
- ↑ a b Robert C. Weast (ed.): CRC Handbook of Chemistry and Physics . 58th edition. CRC Press, Cleveland 1977, ISBN 0-8493-0458-X .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-25.
- ↑ Wiesmann, M .: "Propylene glycol - Ashland and Cargill form a joint venture" , Vogel-Media, Process May 18, 2007.
- ↑ Richard Kuhn, Kichang Kum: About the absolute configuration of sorbin oil. In: Chemical Reports. 95, 1962, pp. 2009-2011, doi: 10.1002 / cber.19620950823 .
- ↑ BASF - 1,2-Propanediol USP (pharmaceutical grade product description), data taken from: Horsley, LH “Azeotropic Data III”, Advances in Chemistry Series, No. 116, ACS, Washington, DC, 1973.
- ^ A b E. Brandes, W. Möller: Safety-related parameters - Volume 1: Flammable liquids and gases , Wirtschaftsverlag NW - Verlag für neue Wissenschaft GmbH, Bremerhaven 2003.
- ↑ Carl J. Sullivan: Propanediols . In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley Online Library, 2012, pp. 213-278 , doi : 10.1002 / 14356007.a22_163 .
- ↑ a b c Federal Institute for Risk Assessment (Ed.): Liquids from e-cigarettes can affect health . Opinion No. 016/2012 of the BfR of February 24, 2012, amended on January 21, 2013. January 21, 2013, p. 3–4 ( bund.de [PDF; 83 kB ; accessed on December 30, 2019]).
- ↑ Clark, et al. Toxicological assessment of heat transfer fluids proposed for use in solar energy applications. Toxicol Appl Pharmac, 51, 529-535., 1979; quoted in: Organization for Economic Cooperation and Development (OECD) "Screening Information Data Set for High Production Volume Chemicals (SIDS)", [1]
- ↑ Gaunt, IF, Carpanini, FMB, Grasso, P and Lansdown, ABG, Long-term toxicity of propylene glycol in rats. Fd Cosmet Toxicol, 10, 151-162, 1972; quoted in: Organization for Economic Cooperation and Development (OECD) "Screening Information Data Set for High Production Volume Chemicals (SIDS)", [2]
- ↑ Murman, test of the acute irritative effects of 1,2-propylene glycol on the eyes and mucous membranes. Huels study no 0212, 1984; Murman, Examination of the Acute Skin Irritant Effects of 1,2-Propylene Glycol. Huels study no 021184. 1984.
- ↑ Clark, et al. Toxicological assessment of heat transfer fluids proposed for use in solar energy applications. Toxicol Appl Pharmac, 51, 529-535., 1979; quoted in: Organization for Economic Cooperation and Development (OECD) "Screening Information Data Set for High Production Volume Chemicals (SIDS)", [3]
- ↑ G. Wieslander: Experimental exposure to propylene glycol mist in aviation emergency training: acute ocular and respiratory effects In: Occup Environ Med . 58 (10), pp. 649-655 (2001) PMID 11555686 .
- ↑ Proposal for labeling propanediol on the ECHA website , accessed on April 25, 2016.
- ↑ Appendix to the ECHA notification of December 13, 2016 , accessed on December 14, 2016.
- ↑ Clariant - 1,2-propylene glycol / water as heat transfer medium ( Memento from August 13, 2014 in the Internet Archive )
- ↑ What's really in my cigarette? BMEL website , accessed on May 25, 2016.
- Jump up ↑ Jens Schubert, Jürgen Hahn, Gerhard Dettbarn, Albrecht Seidel, Andreas Luch, Thomas G. Schulz: Mainstream Smoke of the Waterpipe: Does this Environmental Matrix Reveal as Significant Source of Toxic Compounds. In: Toxicology Letters . 205, 2011, pp. 279-284, doi: 10.1016 / j.toxlet.2011.06.017 .
- ↑ Humectants in water pipe tobacco increase the health risk. BfR, press release of August 3, 2011.
- ↑ Annette Menke: Milk performance feed in the test. In: riswick.de , July 11, 2005.
- ↑ Ch. Noli, F. Scarampella: Hyperkeratosis of the pads . In: Practical Dermatology in Dogs and Cats. Schlütersche Verlagsanstalt, 2nd edition 2005. ISBN 3-87706-713-1 , p. 328.
