Physics
- This is a discussion of a present category of science. For the work by Aristoteles, see “Physics (Aristotle)”.
Physics (Greek: φύσις (phúsis), "nature" and φυσικῆ (phusiké), "knowledge of nature") is the branch of science concerned with discovering and characterizing universal laws that govern matter, energy, space, and time. Discoveries in physics resonate throughout the natural sciences, and physics has been described as the "fundamental science" because other fields such as chemistry and biology investigate systems whose properties depend on the laws of physics.[1]
The emergence of physics as a science distinct from natural philosophy began with the scientific revolution of the 16th and 17th centuries and continued through the dawn of modern physics in the early 20th century. The field has continued to expand, with a growing body of research leading to discoveries such as the Standard Model of fundamental particles and a detailed history of the universe, along with revolutionary new technologies like nuclear weapons and semiconductors. Research today progresses on a vast array of topics, including high-temperature superconductivity, quantum computing, the search for the Higgs boson, and the attempt to develop a theory of quantum gravity. Grounded in observations and experiments and supported by deep, far-reaching theories, physics has made a multitude of contributions to science, technology, and philosophy.
Core Theories
Although physics encompasses a wide variety of phenomena, all physicists are familiar with the basic theories of classical mechanics, electromagnetism, thermodynamics, quantum mechanics, and relativity. Each of these theories has been tested in numerous experiments and proven to be an accurate model of nature within its domain of validity. For example, classical mechanics correctly describes the motion of objects in everyday experience, but it breaks down at the atomic scale, where it is superseded by quantum mechanics, and at speeds approaching the speed of light, where relativistic effects become important. The basic theories form a foundation for the study and research of more specialized topics. A table of these theories, along with many of the concepts they employ, can be found here.
Classical Mechanics

Classical mechanics is a model of the physics of forces acting upon bodies. It is often referred to as "Newtonian mechanics" after Newton and his laws of motion. Classical mechanics is subdivided into statics, which models objects at rest, kinematics, which models objects in motion, and dynamics, which models objects subjected to forces. A recent subdiscipline of dynamics is nonlinear dynamics, the study of systems in which small changes in a variable may have large effects. The science of mechanics may also be broken down, according to the state of matter being studied, into solid mechanics and fluid mechanics. The latter, the mechanics of liquids and gases, includes hydrostatics, hydrodynamics, pneumatics, aerodynamics, and other fields.
Classical mechanics produces very accurate results within the domain of everyday experience. It is superseded by relativistic mechanics for systems moving at large velocities near the speed of light, quantum mechanics for systems at small distance scales, and relativistic quantum field theory for systems with both properties. Nevertheless, classical mechanics is still very useful, because it is much simpler and easier to apply than these other theories, and it has a very large range of approximate validity. Classical mechanics can be used to describe the motion of human-sized objects (such as tops and baseballs), many astronomical objects (such as planets and galaxies), and certain microscopic objects (such as organic molecules.)
An important concept of mechanics is the identification of conserved energy and momentum, which lead to the Lagrangian and Hamiltonian reformulations of Newton's laws. Theories such as fluid mechanics and the kinetic theory of gases result from applying classical mechanics to macroscopic systems. Newton's law of universal gravitation, formulated within classical mechanics, explained Kepler's laws of planetary motion and helped make classical mechanics an important element of the Scientific Revolution.
Electromagnetism

Electromagnetism describes the interaction of charged particles with electric and magnetic fields. It can be divided into electrostatics, the study of interactions between electric charges at rest, and electrodynamics, the study of interactions between moving charges and radiation. The classical theory of electromagnetism is based on the Lorentz force law and Maxwell's equations.
Electrostatics is the study of phenomena associated with charged bodies at rest. Such bodies exert forces on each other, as described by Coulomb’s law, and their behavior can be analyzed in terms of the concept of an electric field surrounding any charged body such that another charged body located at any point in the field is subject to a force proportional to the magnitude of its charge and its attraction or repulsion, depending on the polarity of the charge. Electrostatics has many applications, ranging from the analysis of phenomena such as thunderstorms to the study of the behavior of electron tubes.
Electrodynamics is the study of phenomena associated with charged bodies in motion interacting with varying electric and magnetic fields. Since a moving charge produces a magnetic field, electrodynamics is concerned with effects such as magnetism, electromagnetic radiation, and electromagnetic induction, including such practical applications as the electric generator and the electric motor. This area of electrodynamics, known as classical electrodynamics, was first systematically explained by James Clerk Maxwell, and Maxwell’s equations describe the phenomena of this area with great generality. A more recent development is quantum electrodynamics, which incorporates the laws of quantum theory in order to explain the interaction of electromagnetic radiation with matter. Dirac, Heisenberg, and Pauli were pioneers in the formulation of quantum electrodynamics. Relativistic electrodynamics accounts for relativistic corrections to the motions of charged particles when their speeds approach the speed of light. It applies to phenomena involved with particle accelerators and electron tubes carrying high voltages and currents.
Electromagnetism encompasses various real-world electromagnetic phenomena. For example, light is an oscillating electromagnetic field that is radiated from accelerating charged particles. Aside from gravity, most of the forces in everyday experience are ultimately a result of electromagnetism.
The principles of electromagnetism find applications in various allied disciplines such as microwaves, antennas, electric machines, satellite communications, bioelectromagnetics, plasmas, nuclear research, fiber optics, electromagnetic interference and compatibility, electromechanical energy conversion, radar meteorology, and remote sensing. Electromagnetic devices include transformers, electric relays, radio/TV, telephones, electric motors, transmission lines, waveguides, optical fibers, and lasers.
Thermodynamics and Statistical Mechanics
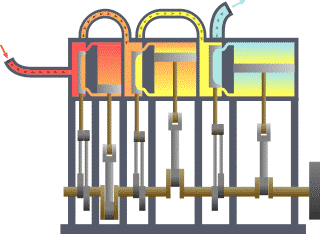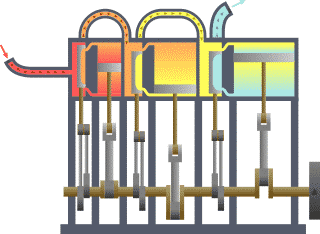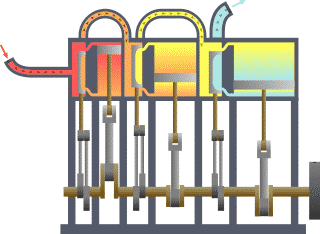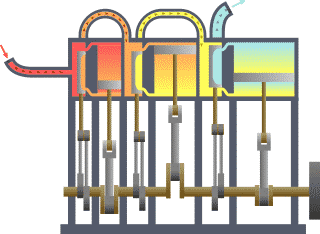
Thermodynamics studies the effects of changes in temperature, pressure, and volume on physical systems at the macroscopic scale by analyzing the collective motion of their particles using statistics.[2][3] Roughly, heat means "energy in transit" and dynamics relates to "movement"; thus, in essence thermodynamics studies the movement of energy and how energy instills movement. Historically, thermodynamics developed out of need to increase the efficiency of early steam engines.[4]
The starting point for most thermodynamic considerations are the laws of thermodynamics, which postulate that energy can be exchanged between physical systems as heat or work.[5] They also postulate the existence of a quantity named entropy, which can be defined for any system.[6] In thermodynamics, interactions between large ensembles of objects are studied and categorized. Central to this are the concepts of system and surroundings. A system is composed of particles, whose average motions define its properties, which in turn are related to one another through equations of state. Properties can be combined to express internal energy and thermodynamic potentials, which are useful for determining conditions for equilibrium and spontaneous processes.
Statistical mechanics analyzes macroscopic systems by applying statistical principles to their microscopic constituents. It provides a framework for relating the microscopic properties of individual atoms and molecules to the macroscopic or bulk properties of materials that can be observed in everyday life, therefore explaining thermodynamics as a natural result of statistics and mechanics (classical and quantum) at the microscopic level. In particular, it can be used to calculate the thermodynamic properties of bulk materials from the spectroscopic data of individual molecules.
Quantum mechanics

Quantum mechanics is the branch of mathematical physics treating atomic and subatomic systems and their interaction with radiation in terms of observable quantities. It is based on the observation that all forms of energy are released in discrete units or bundles called "quanta". Remarkably, quantum theory typically permits only probable or statistical calculation of the observed features of subatomic particles, understood in terms of wavefunctions. The Schrödinger equation plays the role in quantum mechanics that Newton's laws and conservation of energy serve in classical mechanics -- i.e., it predicts the future behavior of a dynamic system -- and is a wave equation in terms of the wavefunction which predicts analytically and precisely the probability of events or outcomes; the detailed outcome is not strictly determined, but given a large number of events, the Schrödinger equation will predict the distribution of results.
According to the older theories of classical physics, energy is treated solely as a continuous phenomenon, while matter is assumed to occupy a very specific region of space and to move in a continuous manner. According to the quantum theory, energy is held to be emitted and absorbed in tiny, discrete amounts. An individual bundle or packet of energy, called a quantum (pl. quanta), thus behaves in some situations much like particles of matter; particles are found to exhibit certain wavelike properties when in motion and are no longer viewed as localized in a given region but rather as spread out to some degree. For example, the light or other radiation given off or absorbed by an atom has only certain frequencies (or wavelengths), as can be seen from the line spectrum associated with the chemical element represented by that atom. The quantum theory shows that those frequencies correspond to definite energies of the light quanta, or photons, and result from the fact that the electrons of the atom can have only certain allowed energy values, or levels; when an electron changes from one allowed level to another, a quantum of energy is emitted or absorbed whose frequency is directly proportional to the energy difference between the two levels.
The restriction of the energy levels of the electrons is explained in terms of the wavelike properties of their motions: electrons occupy only those orbits for which their associated wave is a standing wave (i.e., the circumference of the orbit is exactly equal to a whole number of wavelengths) and thus can have only those energies that correspond to such orbits. Moreover, the electrons are no longer thought of as being at a particular point in the orbit but rather as being spread out over the entire orbit. Just as the results of relativity approximate those of Newtonian physics when ordinary speeds are involved, the results of the quantum theory agree with those of classical physics when very large “quantum numbers” are involved, i.e., on the ordinary large scale of events; this agreement in the classical limit is required by the correspondence principle of Niels Bohr. The quantum theory thus proposes a dual nature for both waves and particles (or a "wave-particle duality"), one aspect predominating in some situations, the other predominating in other situations.
The quantum theory was developed principally over a period of thirty years through the efforts of many scientists. The first contribution was the explanation of black body radiation in 1900 by Max Planck, who proposed that the energies of any harmonic oscillator, such as the atoms of a black body radiator, are restricted to certain values, each of which is an integral (whole number) multiple of a basic, minimum value. The energy E of this basic quantum is directly proportional to the frequency nu of the oscillator, or E=hnu, where h is a constant, now called Planck’s constant, having the value 6.63×10-34 joule-second. In 1905, Albert Einstein proposed that the radiation itself is also quantized according to this same formula, and he used the new theory to explain the photoelectric effect. Following the discovery of the nuclear atom by Rutherford (1911), Bohr used the quantum theory in 1913 to explain both atomic structure and atomic spectra, showing the connection between the electrons’ energy levels and the frequencies of light given off and absorbed.
Quantum mechanics, the final mathematical formulation of the quantum theory, was developed during the 1920s. In 1924, Louis de Broglie proposed that not only do light waves sometimes exhibit particlelike properties, as in the photoelectric effect and atomic spectra, but particles may also exhibit wavelike properties. Two different formulations of quantum mechanics were presented following de Broglie’s suggestion. The wave mechanics of Erwin Schrödinger (1926) involves the use of a mathematical entity, the wave function, which is related to the probability of finding a particle at a given point in space. The matrix mechanics of Werner Heisenberg (1925) makes no mention of wave functions or similar concepts but was shown to be mathematically equivalent to Schrödinger’s theory. A particularly important discovery of the quantum theory is the uncertainty principle, enunciated by Heisenberg in 1927, which places an absolute theoretical limit on the accuracy of certain measurements; as a result, the assumption by earlier scientists that the physical state of a system could be measured exactly and used to predict future states had to be abandoned. Quantum mechanics was combined with the theory of relativity in the formulation of P. A. M. Dirac (1928), which, in addition, predicted the existence of antiparticles. Other developments of the theory include quantum statistics, presented in one form by Einstein and S. N. Bose (the Bose-Einstein statistics) and in another by Dirac and Enrico Fermi (the Fermi-Dirac statistics); quantum electrodynamics, concerned with interactions between charged particles and electromagnetic fields; its generalization, quantum field theory; and quantum electronics. The discovery of quantum mechanics in the early 20th century revolutionized physics, and quantum mechanics is fundamental to most areas of current research.
Relativity
Relativity is a generalization of classical mechanics that describes fast-moving or very massive systems. It includes special and general relativity.
The theory of special relativity was proposed in 1905 by Albert Einstein in his article "On the Electrodynamics of Moving Bodies". It is based on two postulates:
- The mathematical forms of the laws of physics are invariant in all inertial systems.
- The speed of light in a vacuum is constant and independent of the source or observer.
Reconciling the two postulates requires a unification of space and time into the frame-dependent concept of spacetime.
Special relativity has a variety of surprising consequences that seem to violate common sense, but all have been experimentally verified. It overthrows Newtonian notions of absolute space and time by stating that distance and time depend on the observer, and that time and space are perceived differently, depending on the observer. The theory leads to the assertion of change in mass, dimension, and time with increased velocity. It also yields the equivalence of matter and energy, as expressed in the mass-energy equivalence formula E = mc², where c is the speed of light in a vacuum. Special relativity and the Galilean relativity of Newtonian mechanics agree when velocities are small compared to the speed of light.
General relativity is the geometrical theory of gravitation published by Albert Einstein in 1915/16.[7][8] It unifies special relativity, Newton's law of universal gravitation, and the insight that gravitational acceleration can be described by the curvature of space and time by extending special relativity to include transformations between non-inertial frames. In general relativity, the curvature of space-time is produced by the energy of matter and radiation. General relativity is distinguished from other metric theories of gravitation by its use of the Einstein field equations to relate space-time content and space-time curvature. Local Lorentz Invariance requires that the manifolds described in GR be 4-dimensional and Lorentzian instead of Riemannian. In addition, the principle of general covariance forces that mathematics to be expressed using tensor calculus.
The first success of general relativity was in explaining the anomalous perihelion precession of Mercury. Then in 1919, Sir Arthur Eddington announced that observations of stars near the eclipsed Sun confirmed general relativity's prediction that massive objects bend light. Since then, many other observations and experiments have confirmed many of the predictions of general relativity, including gravitational time dilation, the gravitational redshift of light, signal delay, and gravitational radiation. In addition, numerous observations are interpreted as confirming one of general relativity's most mysterious and exotic predictions, the existence of black holes.
Research
Contemporary research in physics can be broadly divided into condensed matter physics; atomic, molecular, and optical physics; particle physics; and astrophysics. Since the twentieth century, the individual fields of physics have become increasingly specialized, and today most physicists work in a single field for their entire careers. "Universalists" such as Albert Einstein (1879–1955) and Lev Landau (1908–1968), who worked in multiple fields of physics, are now very rare. A table of the major fields of physics, along with their subfields and the theories they employ can be found here.
Theory and experiment
The culture of physics research differs from most sciences in the separation of theory and experiment. Since the twentieth century, most individual physicists have specialized in either theoretical physics or experimental physics. The great Italian physicist Enrico Fermi (1901–1954), who made fundamental contributions to both theory and experimentation in nuclear physics, was a notable exception. In contrast, almost all the successful theorists in biology and chemistry (e.g. American quantum chemist and biochemist Linus Pauling) have also been experimentalists, although this is changing as of late.
Theorists seek to develop mathematical models that both agree with existing experiments and successfully predict future results, while experimentalists devise and perform experiments to test theoretical predictions and explore new phenomena. Although theory and experiment are developed separately, they are strongly dependent upon each other. Progress in physics frequently comes about when experimentalists make a discovery that existing theories cannot explain, or when new theories generate experimentally testable predictions. Theorists working closely with experimentalists frequently employ phenomenology.
Theoretical physics is closely related to mathematics, which provides the language of physical theories, and large areas of mathematics, such as calculus, have been invented specifically to solve problems in physics. Theorists may also rely on numerical analysis and computer simulations, which play an ever richer role in the formulation of physical models. The fields of mathematical and computational physics are active areas of research. Theoretical physics sometimes relates to philosophy and metaphysics when it deals with speculative ideas like multidimensional spaces and parallel universes.
Experimental physics is closely related to engineering and technology. Experimental physicists involved in basic research design and perform experiments with equipment such as particle accelerators and lasers, whereas those involved in applied research often work in industry, developing technologies such as magnetic resonance imaging (MRI) and transistors.
Condensed matter

Condensed matter physics is the field of physics that deals with the macroscopic physical properties of matter. In particular, it is concerned with the "condensed" phases that appear whenever the number of constituents in a system is extremely large and the interactions between the constituents are strong. The most familiar examples of condensed phases are solids and liquids, which arise from the bonding and electromagnetic force between atoms. More exotic condensed phases include the superfluid and the Bose-Einstein condensate found in certain atomic systems at very low temperatures, the superconducting phase exhibited by conduction electrons in certain materials, and the ferromagnetic and antiferromagnetic phases of spins on atomic lattices.
Condensed matter physics is by far the largest field of contemporary physics. Much progress has also been made in theoretical condensed matter physics. By one estimate, one third of all American physicists identify themselves as condensed matter physicists. Historically, condensed matter physics grew out of solid-state physics, which is now considered one of its main subfields. The term "condensed matter physics" was apparently coined by Philip Anderson when he renamed his research group - previously "solid-state theory" - in 1967. In 1978, the Division of Solid State Physics at the American Physical Society was renamed as the Division of Condensed Matter Physics.[9] Condensed matter physics has a large overlap with chemistry, materials science, nanotechnology and engineering.
Atomic, molecular, and optical

Atomic, molecular, and optical physics (AMO) is the study of matter-matter and light-matter interactions on the scale of single atoms or structures containing a few atoms. The three areas are grouped together because of their interrelationships, the similarity of methods used, and the commonality of the energy scales that are relevant. All three areas include both classical and quantum treatments.
Atomic physics studies the electron hull of atoms. This branch of physics is distinct from nuclear physics, despite their association in the public consciousness. Atomic physics is not concerned with the intra-nuclear processes studied in nuclear physics, although properties of the nucleus can be important in atomic physics (e.g., hyperfine structure). Current research focuses on activities in quantum control, cooling and trapping of atoms and ions, low-temperature collision dynamics, the collective behavior of atoms in weakly interacting gases (Bose-Einstein Condensates and dilute Fermi degenerate systems), precision measurements of fundamental constants, and the effects of electron correlation on structure and dynamics.
Molecular physics focuses on multi-atomic structures and their internal and external interactions with matter and light. Optical physics is distinct from optics in that it tends to focus not on the control of classical light fields by macroscopic objects, but on the fundamental properties of optical fields and their interactions with matter in the microscopic realm.
High energy
Particle physics is the study of elementary constituents of matter and radiation, and the interactions between them. It is also called "high energy physics", because many elementary particles do not occur under normal circumstances in nature, but can be created and detected during energetic collisions of other particles, as is done in particle accelerators.
The current state of the classification of elementary particles is the Standard Model. It describes the strong, weak, and electromagnetic fundamental forces, using mediating gauge bosons. The species of gauge bosons are the gluons, W- and W+ and Z bosons, and the photon, respectively. The model also contains 24 fundamental particles (12 particle/anti-particle pairs), which are the constituents of matter. Finally, it predicts the existence of a type of boson known as the Higgs boson, which has yet to be discovered.
Astrophysics

Astrophysics is the application of the theories and methods of physics to the study of stellar structure, stellar evolution, the origin of the solar system, and related problems of cosmology. Because astrophysics is a very broad subject, astrophysicists typically apply many disciplines of physics, including mechanics, electromagnetism, statistical mechanics, thermodynamics, quantum mechanics, relativity, nuclear and particle physics, and atomic and molecular physics. The distinction between astrophysics and modern astronomy is disappearing in scientific usage.
Astronomy is one of the oldest sciences. Astronomers of early civilizations performed methodical observations of the night sky, and astronomical artifacts have been found from much earlier periods. Thales, an Ionian philosopher of the 6th cent. B.C., is credited with introducing geometrical ideas into astronomy. About a hundred years later Pythagorus imagined the universe as a series of concentric spheres and Eudoxus introduced the idea of rotating spheres to account for the observed complexities of the planets' motions. Hipparchus developed trigonometry and applied it to astronomy and later Ptolemy created a scheme of epicycles and a parallax technique. European astronomy lay dormant for fourteen centuries until Nicholaus Copernicus retained the uniform circular motion of the Ptolemaic system in his system, but by placing the sun at the center, he was able to reduce the number of epicycles. The observations of Tycho Brahe led Johannes Kepler to the three laws of planetary motion that bear his name (see Kepler's laws of planetary motion). The invention of the telescope, which was first applied to astronomy by Galileo, helped the discipline develop into a modern science, which is today often referred to as astrophysics. Isaac Newton succeeded in uniting the sciences of astronomy and physics. His laws of motion and theory of universal gravitation provided a physical, dynamic basis for the merely descriptive laws of Kepler. Celestial mechanics, the application of Newtonian gravity and Newton's laws to explain Kepler's laws of planetary motion, was the first unification of astronomy and physics. By the early 19th cent., the science of celestial mechanics had reached a highly developed state at the hands of Leonhard Euler, J. L. Lagrange, P. S. Laplace, and others. Powerful new mathematical techniques allowed solution of most of the remaining problems in classical gravitational theory as applied to the solar system.
At the end of the 19th century, it was discovered that, when decomposing the light from the Sun, a multitude of spectral lines were observed (regions where there was less or no light), which would go on to revolutionize astronomy. Experiments with hot gases showed that the same lines could be observed in the spectra of gases, specific lines corresponding to unique chemical elements. In this way it was proved that the chemical elements found in the Sun (chiefly hydrogen) were also found on Earth. Indeed, the element helium was first discovered in the spectrum of the Sun and only later on Earth, hence its name. During the 20th century, spectroscopy (the study of these spectral lines) advanced, particularly as a result of the advent of quantum physics that was necessary to understand the astronomical and experimental observations.[10] Interest shifted from determining the positions and distances of stars to studying their physical composition (see stellar structure and stellar evolution).
Physical cosmology is the study of the formation and evolution of the universe and represents astrophysics on its largest scales. Albert Einstein’s theory of relativity plays a central role in all modern cosmological theories. In the early 20th century, Hubble's discovery that the universe was expanding, as shown by the Hubble diagram, led to the Big Bang theory of cosmology which was confirmed by the success of Big Bang nucleosynthesis and the discovery of the cosmic microwave background in 1964. The Big Bang model rests on two theoretical pillars: Albert Einstein's general relativity and the cosmological principle.[11] Cosmologists have recently established a precise model of the evolution of the universe, which include cosmic inflation, dark energy and dark matter.
The discovery by Karl Jansky in 1931 that radio signals were emitted by celestial bodies initiated the science of radio astronomy. Most recently, the frontiers of astronomy have been expanded by space exploration. Perturbations and interference from the earth’s atmosphere make space-based observations necessary for infrared, ultraviolet, gamma-ray, and X-ray astronomy. The Hubble Space Telescope, launched in 1990, has made possible visual observations of a quality far exceeding those of earthbound instruments.
Applied physics
Applied physics is physics that is intended for a particular technological or practical use, as for example in engineering, as opposed to basic research. Applied physics is rooted in the fundamental truths and basic concepts of the physical sciences, but is concerned with the use of scientific principles in practical devices and systems, and in the application of physics in other areas of science. The approach is similar to that of applied mathematics. "Applied" is distinguished from "pure" by a subtle combination of factors such as the motivation and attitude of researchers and the nature of the relationship to the technology or science that may be affected by the work.
Notes
- ^ The Feynman Lectures on Physics Volume I, Chapter III. Feynman, Leighton and Sands. ISBN 0-201-02115-3 For the philosophical issues of whether other sciences can be "reduced" to physics, see reductionism and special sciences.
- ^ Perrot, Pierre (1998). A to Z of Thermodynamics. Oxford University Press. ISBN 0-19-856552-6.
- ^ Clark, John, O.E. (2004). The Essential Dictionary of Science. Barnes & Noble Books. ISBN 0-7607-4616-8.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Clausius, Ruldolf (1850). On the Motive Power of Heat, and on the Laws which can be deduced from it for the Theory of Heat. Poggendorff's Annalen der Physick, LXXIX (Dover Reprint). ISBN 0-486-59065-8.
{{cite book}}: Italic or bold markup not allowed in:|publisher=(help) - ^ Van Ness, H.C. (1969). Understanding Thermodynamics. Dover Publications, Inc. ISBN 0-486-63277-6.
- ^ Dugdale, J.S. (1998). Entropy and its Physical Meaning. Taylor and Francis. ISBN 0-7484-0569-0.
- ^ Einstein, Albert (November 25, 1915). "Die Feldgleichungun der Gravitation". Sitzungsberichte der Preussischen Akademie der Wissenschaften zu Berlin: 844–847. Retrieved 2006-09-12.
{{cite journal}}: Check date values in:|date=(help) - ^ Einstein, Albert (1916). "The Foundation of the General Theory of Relativity" (PDF). Annalen der Physik. Retrieved 2006-09-03.
- ^ "Division of Condensed Matter Physics Governance History". Retrieved 2007-02-13.
- ^ Frontiers of Astrophysics: Workshop Summary, H. Falcke, P. L. Biermann
- ^ http://map.gsfc.nasa.gov/m_uni/uni_101bb1.html
Further reading
- A large number of textbooks, popular books, and webpages about physics are available for further reading.
- Important publications in physics
- History of physics
Organizations
- AIP.org is the website of the American Institute of Physics
- IOP.org is the website of the Institute of Physics
- APS.org is the website of the American Physical Society
- SPS National is the website of the American Society of Physics Students
- CAP.ca is the website of the Canadian Association of Physicists
- EPS.org is the website of the European Physical Society
References
- Yang, Mills 1954 Physical Review 95, 631; Yang, Mills 1954 Physical Review 96, 191.
