Tibrovirus: Difference between revisions
Mallardrise (talk | contribs) No edit summary |
No edit summary Tags: Mobile edit Mobile web edit |
||
| (23 intermediate revisions by 12 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Genus of viruses}} |
|||
{{Use dmy dates|date=April 2017}} |
{{Use dmy dates|date=April 2017}} |
||
{{Virusbox |
|||
{{taxobox |
|||
| image = Sweetwater branch virus.jpg |
|||
| ⚫ | |||
| |
| image_alt = |
||
| image_caption = ''[[Sweetwater Branch tibrovirus]]'' (530 nm to 690 nm and up to 900 nm long, 65 nm to 75 nm in diameter)<ref name=":3">Popov VL, Tesh RB, Weaver SC, Vasilakis N. Electron Microscopy in Discovery of Novel and Emerging Viruses from the Collection of the World Reference Center for Emerging Viruses and Arboviruses (WRCEVA). ''Viruses''. 2019;11(5):477. Published 2019 May 25. doi:10.3390/v11050477</ref> |
|||
| ordo = ''[[Mononegavirales]]'' |
|||
| ⚫ | |||
| familia = ''[[Rhabdoviridae]]'' |
|||
| ⚫ | |||
| genus = '''''Tibrovirus''''' |
|||
| subdivision_ref = <ref>{{cite web |title=Virus Taxonomy: 2018b Release |url=https://ictv.global/taxonomy |website=International Committee on Taxonomy of Viruses (ICTV) |accessdate=3 February 2020 |language=en |date=March 2019}}</ref> |
|||
| ⚫ | |||
| subdivision = |
|||
* |
|||
| ⚫ | |||
* ''[[Bas-Congo tibrovirus]]'' |
|||
| subdivision = |
|||
*''Beatrice Hill |
* ''[[Beatrice Hill tibrovirus]]'' |
||
*''Coastal Plains tibrovirus'' |
* ''[[Coastal Plains tibrovirus]]'' |
||
*'' |
* ''[[Ekpoma 1 tibrovirus]]'' |
||
* ''[[Ekpoma 2 tibrovirus]]'' |
|||
* ''[[Sweetwater Branch tibrovirus]]'' |
|||
| ⚫ | |||
| synonyms = |
|||
| synonyms_ref = |
|||
}} |
}} |
||
'''''Tibrovirus''''' is a genus of [[ |
'''''Tibrovirus''''' is a poorly characterized genus of [[virus]]es in the family ''[[Rhabdoviridae]]'', order ''[[Mononegavirales]]''. There are 8 members of the genus.<ref>{{Cite web|url=https://talk.ictvonline.org/ictv-reports/ictv_online_report/negative-sense-rna-viruses/mononegavirales/w/rhabdoviridae/802/genus-tibrovirus|title=International Committee on the Taxonomy of Viruses - Tibrovirus genus|last=|first=|date=|website=|url-status=dead|archive-url=https://web.archive.org/web/20190808211734/https://talk.ictvonline.org/ictv-reports/ictv_online_report/negative-sense-rna-viruses/mononegavirales/w/rhabdoviridae/802/genus-tibrovirus|archive-date=8 August 2019|access-date=}}</ref> Tibroviruses have been isolated from biting midges, cattle, and humans. None of the tibroviruses, except for Bas-Congo virus, have been associated with any diseases. |
||
== |
==Genus members== |
||
* '''''[[Bas-Congo virus]]''''' (BASV) was discovered in 2009 in the Democratic Republic of Congo in a blood sample collected from a 32-year-old male who survived a severe illness resembling hemorrhagic fever.<ref>Grard G, Fair JN, Lee D, et al. A novel rhabdovirus associated with acute hemorrhagic fever in central Africa [published correction appears in PLoS Pathog. 2016 Mar;12(3):e1005503] [published correction appears in PLoS Pathog. 2017 Sep 7;13(9):e1006583]. ''PLoS Pathog''. 2012;8(9):e1002924. doi:10.1371/journal.ppat.1002924</ref> BASV could not be isolated from the patient’s sample has not been established as a human pathogen.<ref>{{Cite web | url=https://science.sciencemag.org/content/bas-congo-virus-not-established-pathogen |title = Bas-Congo virus - not an established pathogen|date = 3 December 2018|last1 = Branco|first1 = Luis M.|last2 = Garry|first2 = Robert F.|accessdate=31 January 2020}}</ref> |
|||
* '''''[[Beatrice Hill virus]]''''' (BHV) was isolated from a pool of biting midges (Culicoides peregrinus) in 1984 in Northern Territory, Australia.<ref>Standfast, H. A.; Dyce, A. L.; St George, T. D.; Muller, M. J.; Doherty, R. L.; Carley, J. G.; Filippich, C., Isolation of arboviruses from insects collected at Beatrice Hill, Northern Territory of Australia, 1974-1976. ''Aust J Biol Sci'' '''1984,''' 37, (5-6), 351-66 </ref> BHV is poorly characterized and serological studies to assess its prevalence have not been carried out. |
|||
* '''''[[Bivens arm virus]]''''' (BAV) was isolated in 1981-1982 from a pool of biting midges in Florida. Anti-BAV antibodies have been detected in a variety of animals, including cattle, throughout Florida and the Caribbean.<ref name=":0">Gibbs, E. P.; [[Charles Calisher|Calisher, C. H.]]; Tesh, R. B.; Lazuick, J. S.; Bowen, R.; Greiner, E. C., Bivens Arm virus: a new rhabdovirus isolated from ''Culicoides insignis'' in Florida and related to Tibrogargan virus of Australia. ''Vet Microbiol'' '''1989,''' 19, (2), 141-50 </ref> There is no evidence of human infection or any disease associated with BAV.<ref name=":0" /> |
|||
| ⚫ | * '''''[[Coastal Plains virus]]''''' (CPV) was discovered in 1981 in Northern Territory, Australia in the blood of a healthy, asymptomatic steer.<ref>Cybinski DH, Gard GP. Isolation of a new rhabdovirus in Australia related to Tibrogargan virus. ''Aust J Biol Sci''. 1986;39(3):225–232. doi:10.1071/bi9860225</ref> No anti-CPV antibodies have ever been detected in humans and no disease has been associated with CPV. |
||
* '''''[[Ekpoma virus 1]]''''' (EKV-1) was discovered in 2015 in a blood sample collected from a healthy, 45-year-old woman living in Ekpoma, Nigeria.<ref name=":1">Stremlau MH, Andersen KG, Folarin OA, et al. Discovery of novel rhabdoviruses in the blood of healthy individuals from West Africa. ''PLoS Negl Trop Dis''. 2015;9(3):e0003631. Published 2015 Mar 17. doi:10.1371/journal.pntd.0003631</ref> EKV-1 was present in her blood at 4.5 million RNA copies/mL suggesting robust replication. Experiments using a recombinant vesicular stomatitis virus expressing the EKV-1 envelope glycoprotein indicate EKV-1 has very broad tropism and can efficiently enter nearly all types of human cells.<ref name=":2">Caì Y, Yú S, Jangra RK, et al. Human, Nonhuman Primate, and Bat Cells Are Broadly Susceptible to Tibrovirus Particle Cell Entry. ''Front Microbiol''. 2019;10:856. Published 2019 Apr 26. doi:10.3389/fmicb.2019.00856</ref> EKV-1 could not be isolated from the patient’s blood sample and live replication-competent virus is not available. |
|||
* '''''[[Ekpoma virus 2]]''''' (EKV-2) was discovered in 2015 in a blood sample collected from a healthy, 19-year-old woman living in Ekpoma, Nigeria.<ref name=":1" /> EKV-2 was present in her blood at 45,000 RNA copies/mL suggesting modest replication. Experiments using a recombinant vesicular stomatitis virus expressing the EKV-2 envelope glycoprotein indicate EKV-2 has very broad tropism similar to EKV-1.<ref name=":2" /> EKV-2 also could not be isolated from the patient’s blood sample and live replication-competent virus is not available. |
|||
* '''''[[Sweetwater branch virus]]''''' (SWBV) was isolated along with BAV from pools of biting midges in Florida in 1981-1982. Like BAV, SWBV exposure in various animals is likely widespread. There is no evidence of human exposure or any disease associated with the virus. |
|||
* '''''[[Tibrogargan virus]]''''' (TIBV), the first tibrovirus discovered, was isolated from a pool of biting midges (Culicoides brevitarsis) in 1976 in Peachester, Australia.<ref>Cybinski, D. H.; St. George, T. D.; Standfast, H. A.; McGregor, A., Isolation of Tibrogargan virus, a new Australian rhabdovirus, from ''Culicoides brevitaris''. ''Vet Microbiol'' '''1980,''' 5, 301-308 </ref> TIBV appears to be widespread among cattle in Australia. A survey of more than 3,000 cattle found many herds were 100% seropositive. TIBV infection of humans has not been reported. TIBV is an orphan virus and not associated with any disease. Experimental infections of cattle produced viremia, but no observable signs of illness.<ref>Cybinski, D. H.; Gard, G. P., Isolation of a new rhabdovirus in Australia related to Tibrogargan virus. ''Aust J Biol Sci'' '''1986,''' 39, (3), 225-32 </ref> |
|||
| ⚫ | |||
Tibrogargan virus was the first tibrovirus discovered<ref>Cybinski, D. H., St. George, T. D., Standfast, H. A. & McGregor, A. Isolation of tibrogargan virus, a new Australian rhabdovirus, from Culicoides brevitarsis. Veterinary Microbiology 5, 301–308 (1980)</ref>. It was isolated from a pool of biting midges (''Culicoides brevitaris'') collected in 1976 in Preachester, Australia. |
|||
| ⚫ | |||
==Genetic divergence== |
|||
| ⚫ | |||
Tibroviruses are highly divergent. For example, overall amino acid homology among the human-associated tibroviruses (i.e. BASV, EKV-1 and EKV-2) ranges from 33% - 39%.<ref name=":1" /> |
|||
Between 1981-1982, two tibroviruses were isolated from biting midges in Florida<ref>Gibbs, E. P. et al. Bivens arm virus: a new rhabdovirus isolated from Culicoides insignis in Florida and related to Tibrogargan virus of Australia. Vet. Microbiol. 19, 141–150 (1989)</ref>. These midges were feeding on Water Buffalo imported from Trinidad. |
|||
In 1984, another tibrovirus from Australia was reported<ref>StandJast, H. et al. Isolation of Arboviruses from Insects Collected at Beatrice Hill, Northern Territory of Australia, 1974?1976. Australian Journal of Biological Sciences 37, 351 (1984)</ref>. This tibrovirus was named Beatrice Hill virus. It is 72% identical to Tibrogargan virus and Bivens Arm virus. |
|||
In 2012, a novel tibrovirus called Bas-Congo was detected in the plasma of a patient in the Democratic Republic of Congo suffering from symptoms resembling hemorrhagic fever<ref>Grard, G. et al. A novel rhabdovirus associated with acute hemorrhagic fever in central Africa. PLoS Pathog. 8, (2012)</ref>. The patient recovered from his illness. |
|||
In 2015, two more human-associated tibroviruses were reported<ref>Stremlau, M. H. et al. Discovery of novel rhabdoviruses in the blood of healthy individuals from West Africa. PLoS Negl Trop Dis 9, e0003631 (2015)</ref>. These viruses, called Ekpoma virus 1 and Ekpoma virus 2, were detected in plasma samples from two healthy women in Nigeria. |
|||
| ⚫ | |||
| ⚫ | |||
== Clinical Disease == |
|||
With the exception of Bas-Congo virus, none of the other tibroviruses have been associated with disease. |
|||
Antibodies that neutralize Tibrogargan virus have been found in cattle and buffalo in Australia. Some herd are 100% seropositive. However, Tibrogargan antibodies have never been detected in humans. Furthermore, no disease has been associated with Tibrogargan virus. Cattle have been experimentally inoculated with Tibrovirus, but no overt signs of illness have been observed<ref>Cybinski, D. H., St. George, T. D., Standfast, H. A. & McGregor, A. Isolation of tibrogargan virus, a new Australian rhabdovirus, from Culicoides brevitarsis. Veterinary Microbiology 5, 301–308 (1980)</ref>. |
|||
Bas-Congo virus has been associated with a case of hemorrhagic fever. However, it is not known whether Bas-Congo virus actually caused the patient's illness. |
|||
The Ekpoma viruses are not associated with clinical symptoms in Nigeria. The two viruses were detected in two apparently healthy, asymptomatic women. |
|||
==Morphology== |
==Morphology== |
||
Tibrovirus virions are enveloped and |
Tibrovirus virions are enveloped, but only the morphology of Tibrogargan virus and Sweetwater branch virus have been observed by electron microscopy.<ref name=":3" /> |
||
{| class="wikitable sortable" style="text-align:center" |
{| class="wikitable sortable" style="text-align:center" |
||
| Line 51: | Line 46: | ||
! Genus !! Structure || Symmetry !! Capsid !! Genomic arrangement !! Genomic segmentation |
! Genus !! Structure || Symmetry !! Capsid !! Genomic arrangement !! Genomic segmentation |
||
|- |
|- |
||
|''Tibrovirus''||Bullet-shaped||||Enveloped||Linear|| |
|''Tibrovirus''||Bullet-shaped||Helical||Enveloped||Linear||Non-segmented |
||
|} |
|} |
||
==Genome== |
==Genome== |
||
Tibrovirus genomes are single-stranded, negative-sense RNA molecules approximately 13 kb in length. The genome encodes for the |
Tibrovirus genomes are single-stranded, negative-sense RNA molecules approximately 13 kb in length. The genome encodes for the typical five proteins found in all rhabdoviruses: nucleocapsid (N), phosphoprotein (P), matrix (M), glycoprotein (G), and polymerase (L). However, there are three additional genes, U1-U3, that encode for proteins of unknown function.<ref name=ViralZone>{{cite web|title=Viral Zone|url=http://viralzone.expasy.org/all_by_species/4676.html|publisher=ExPASy|accessdate=13 August 2015}}</ref> |
||
==Life cycle== |
==Life cycle== |
||
Viral replication is cytoplasmic. Entry into the host cell is achieved by attachment of the viral G glycoproteins to host receptors, which mediate clathrin-mediated endocytosis. Replication follows the negative-stranded RNA virus replication model. Negative stranded RNA virus transcription, using polymerase stuttering is the method of transcription. The virus exits the host cell by budding, and tubule-guided viral movement. |
Viral replication is cytoplasmic. Entry into the host cell is achieved by attachment of the viral G glycoproteins to host receptors, which mediate clathrin-mediated endocytosis.<ref>Steffen I, Liss NM, Schneider BS, Fair JN, Chiu CY, Simmons G. Characterization of the Bas-Congo virus glycoprotein and its function in pseudotyped viruses. ''J Virol''. 2013;87(17):9558–9568. doi:10.1128/JVI.01183-13</ref><ref name=":2" /> Replication follows the negative-stranded RNA virus replication model. Negative stranded RNA virus transcription, using polymerase stuttering is the method of transcription. The virus exits the host cell by budding, and tubule-guided viral movement.{{cn|date=November 2022}} |
||
Cattle and water buffalo serve as the natural host.<ref name=ViralZone/> |
|||
{| class="wikitable sortable" style="text-align:center" |
{| class="wikitable sortable" style="text-align:center" |
||
| Line 67: | Line 61: | ||
|''Tibrovirus''||Bovine||None||Clathrin-mediated endocytosis||Budding||Cytoplasm||Cytoplasm||Zoonosis; arthropod bite: midges |
|''Tibrovirus''||Bovine||None||Clathrin-mediated endocytosis||Budding||Cytoplasm||Cytoplasm||Zoonosis; arthropod bite: midges |
||
|} |
|} |
||
==Taxonomy== |
|||
{| class="sortable wikitable" |
|||
|+ Genus ''Tibrovirus'': species and their viruses<ref>{{Cite journal|last=Afonso|first=Claudio L.|last2=Amarasinghe|first2=Gaya K.|last3=Bányai|first3=Krisztián|last4=Bào|first4=Yīmíng|last5=Basler|first5=Christopher F.|last6=Bavari|first6=Sina|last7=Bejerman|first7=Nicolás|last8=Blasdell|first8=Kim R.|last9=Briand|first9=François-Xavier|date=2016-08-01|title=Taxonomy of the order Mononegavirales: update 2016|journal=Archives of Virology|volume=161|issue=8|pages=2351–2360|doi=10.1007/s00705-016-2880-1|issn=1432-8798|pmc=4947412|pmid=27216929}}</ref> |
|||
|- |
|||
| '''[[International Committee on Taxonomy of Viruses|Genus]]''' |
|||
| '''[[International Committee on Taxonomy of Viruses|Species]]''' |
|||
| '''[[International Committee on Taxonomy of Viruses|Virus (Abbreviation)]]''' |
|||
|- valign="TOP" |
|||
| rowspan="3" | ''Tibrovirus'' |
|||
| rowspan="1" | ''Coastal Plains tibrovirus'' |
|||
| rowspan="1" | Coastal Plains virus (CPV) |
|||
|- valign="TOP" |
|||
| rowspan="2" | ''Tibrogargan tibrovirus''* |
|||
| rowspan="1" | Bivens Arm virus (BAV) |
|||
|- valign="TOP" |
|||
| rowspan="1" | Tibrogargan virus (TIBV) |
|||
|- valign="TOP" |
|||
|} |
|||
Table legend: "*" denotes type species. |
|||
==References== |
==References== |
||
| Line 101: | Line 73: | ||
[[Category:Rhabdoviridae]] |
[[Category:Rhabdoviridae]] |
||
[[Category: |
[[Category:Virus genera]] |
||
Latest revision as of 21:47, 23 May 2023
| Tibrovirus | |
|---|---|
| |
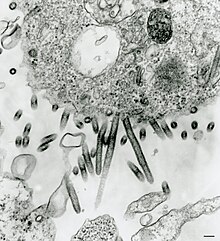
| Sweetwater Branch tibrovirus (530 nm to 690 nm and up to 900 nm long, 65 nm to 75 nm in diameter)[2] | |
| Virus classification | |
| (unranked): | Virus |
| Realm: | Riboviria |
| Kingdom: | Orthornavirae |
| Phylum: | Negarnaviricota |
| Class: | Monjiviricetes |
| Order: | Mononegavirales |
| Family: | Rhabdoviridae |
| Genus: | Tibrovirus |
| Species[1] | |
Tibrovirus is a poorly characterized genus of viruses in the family Rhabdoviridae, order Mononegavirales. There are 8 members of the genus.[3] Tibroviruses have been isolated from biting midges, cattle, and humans. None of the tibroviruses, except for Bas-Congo virus, have been associated with any diseases.
Genus members[edit]
- Bas-Congo virus (BASV) was discovered in 2009 in the Democratic Republic of Congo in a blood sample collected from a 32-year-old male who survived a severe illness resembling hemorrhagic fever.[4] BASV could not be isolated from the patient’s sample has not been established as a human pathogen.[5]
- Beatrice Hill virus (BHV) was isolated from a pool of biting midges (Culicoides peregrinus) in 1984 in Northern Territory, Australia.[6] BHV is poorly characterized and serological studies to assess its prevalence have not been carried out.
- Bivens arm virus (BAV) was isolated in 1981-1982 from a pool of biting midges in Florida. Anti-BAV antibodies have been detected in a variety of animals, including cattle, throughout Florida and the Caribbean.[7] There is no evidence of human infection or any disease associated with BAV.[7]
- Coastal Plains virus (CPV) was discovered in 1981 in Northern Territory, Australia in the blood of a healthy, asymptomatic steer.[8] No anti-CPV antibodies have ever been detected in humans and no disease has been associated with CPV.
- Ekpoma virus 1 (EKV-1) was discovered in 2015 in a blood sample collected from a healthy, 45-year-old woman living in Ekpoma, Nigeria.[9] EKV-1 was present in her blood at 4.5 million RNA copies/mL suggesting robust replication. Experiments using a recombinant vesicular stomatitis virus expressing the EKV-1 envelope glycoprotein indicate EKV-1 has very broad tropism and can efficiently enter nearly all types of human cells.[10] EKV-1 could not be isolated from the patient’s blood sample and live replication-competent virus is not available.
- Ekpoma virus 2 (EKV-2) was discovered in 2015 in a blood sample collected from a healthy, 19-year-old woman living in Ekpoma, Nigeria.[9] EKV-2 was present in her blood at 45,000 RNA copies/mL suggesting modest replication. Experiments using a recombinant vesicular stomatitis virus expressing the EKV-2 envelope glycoprotein indicate EKV-2 has very broad tropism similar to EKV-1.[10] EKV-2 also could not be isolated from the patient’s blood sample and live replication-competent virus is not available.
- Sweetwater branch virus (SWBV) was isolated along with BAV from pools of biting midges in Florida in 1981-1982. Like BAV, SWBV exposure in various animals is likely widespread. There is no evidence of human exposure or any disease associated with the virus.
- Tibrogargan virus (TIBV), the first tibrovirus discovered, was isolated from a pool of biting midges (Culicoides brevitarsis) in 1976 in Peachester, Australia.[11] TIBV appears to be widespread among cattle in Australia. A survey of more than 3,000 cattle found many herds were 100% seropositive. TIBV infection of humans has not been reported. TIBV is an orphan virus and not associated with any disease. Experimental infections of cattle produced viremia, but no observable signs of illness.[12]
Transmission[edit]
BHV, BAV, SWBV and TIBV were isolated from biting midges, suggesting that midges are the major arthropod vector for these viruses. It is not known how BASV, EKV-1 and EKV-2 are transmitted.[citation needed]
Genetic divergence[edit]
Tibroviruses are highly divergent. For example, overall amino acid homology among the human-associated tibroviruses (i.e. BASV, EKV-1 and EKV-2) ranges from 33% - 39%.[9]
Morphology[edit]
Tibrovirus virions are enveloped, but only the morphology of Tibrogargan virus and Sweetwater branch virus have been observed by electron microscopy.[2]
| Genus | Structure | Symmetry | Capsid | Genomic arrangement | Genomic segmentation |
|---|---|---|---|---|---|
| Tibrovirus | Bullet-shaped | Helical | Enveloped | Linear | Non-segmented |
Genome[edit]
Tibrovirus genomes are single-stranded, negative-sense RNA molecules approximately 13 kb in length. The genome encodes for the typical five proteins found in all rhabdoviruses: nucleocapsid (N), phosphoprotein (P), matrix (M), glycoprotein (G), and polymerase (L). However, there are three additional genes, U1-U3, that encode for proteins of unknown function.[13]
Life cycle[edit]
Viral replication is cytoplasmic. Entry into the host cell is achieved by attachment of the viral G glycoproteins to host receptors, which mediate clathrin-mediated endocytosis.[14][10] Replication follows the negative-stranded RNA virus replication model. Negative stranded RNA virus transcription, using polymerase stuttering is the method of transcription. The virus exits the host cell by budding, and tubule-guided viral movement.[citation needed]
| Genus | Host details | Tissue tropism | Entry details | Release details | Replication site | Assembly site | Transmission |
|---|---|---|---|---|---|---|---|
| Tibrovirus | Bovine | None | Clathrin-mediated endocytosis | Budding | Cytoplasm | Cytoplasm | Zoonosis; arthropod bite: midges |
References[edit]
- ^ "Virus Taxonomy: 2018b Release". International Committee on Taxonomy of Viruses (ICTV). March 2019. Retrieved 3 February 2020.
- ^ a b Popov VL, Tesh RB, Weaver SC, Vasilakis N. Electron Microscopy in Discovery of Novel and Emerging Viruses from the Collection of the World Reference Center for Emerging Viruses and Arboviruses (WRCEVA). Viruses. 2019;11(5):477. Published 2019 May 25. doi:10.3390/v11050477
- ^ "International Committee on the Taxonomy of Viruses - Tibrovirus genus". Archived from the original on 8 August 2019.
- ^ Grard G, Fair JN, Lee D, et al. A novel rhabdovirus associated with acute hemorrhagic fever in central Africa [published correction appears in PLoS Pathog. 2016 Mar;12(3):e1005503] [published correction appears in PLoS Pathog. 2017 Sep 7;13(9):e1006583]. PLoS Pathog. 2012;8(9):e1002924. doi:10.1371/journal.ppat.1002924
- ^ Branco, Luis M.; Garry, Robert F. (3 December 2018). "Bas-Congo virus - not an established pathogen". Retrieved 31 January 2020.
- ^ Standfast, H. A.; Dyce, A. L.; St George, T. D.; Muller, M. J.; Doherty, R. L.; Carley, J. G.; Filippich, C., Isolation of arboviruses from insects collected at Beatrice Hill, Northern Territory of Australia, 1974-1976. Aust J Biol Sci 1984, 37, (5-6), 351-66
- ^ a b Gibbs, E. P.; Calisher, C. H.; Tesh, R. B.; Lazuick, J. S.; Bowen, R.; Greiner, E. C., Bivens Arm virus: a new rhabdovirus isolated from Culicoides insignis in Florida and related to Tibrogargan virus of Australia. Vet Microbiol 1989, 19, (2), 141-50
- ^ Cybinski DH, Gard GP. Isolation of a new rhabdovirus in Australia related to Tibrogargan virus. Aust J Biol Sci. 1986;39(3):225–232. doi:10.1071/bi9860225
- ^ a b c Stremlau MH, Andersen KG, Folarin OA, et al. Discovery of novel rhabdoviruses in the blood of healthy individuals from West Africa. PLoS Negl Trop Dis. 2015;9(3):e0003631. Published 2015 Mar 17. doi:10.1371/journal.pntd.0003631
- ^ a b c Caì Y, Yú S, Jangra RK, et al. Human, Nonhuman Primate, and Bat Cells Are Broadly Susceptible to Tibrovirus Particle Cell Entry. Front Microbiol. 2019;10:856. Published 2019 Apr 26. doi:10.3389/fmicb.2019.00856
- ^ Cybinski, D. H.; St. George, T. D.; Standfast, H. A.; McGregor, A., Isolation of Tibrogargan virus, a new Australian rhabdovirus, from Culicoides brevitaris. Vet Microbiol 1980, 5, 301-308
- ^ Cybinski, D. H.; Gard, G. P., Isolation of a new rhabdovirus in Australia related to Tibrogargan virus. Aust J Biol Sci 1986, 39, (3), 225-32
- ^ "Viral Zone". ExPASy. Retrieved 13 August 2015.
- ^ Steffen I, Liss NM, Schneider BS, Fair JN, Chiu CY, Simmons G. Characterization of the Bas-Congo virus glycoprotein and its function in pseudotyped viruses. J Virol. 2013;87(17):9558–9568. doi:10.1128/JVI.01183-13
