3,5-diiodosalicylic acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | 3,5-diiodosalicylic acid | |||||||||||||||||||||
| other names |
3,5-diiodo-2-hydroxybenzoic acid |
|||||||||||||||||||||
| Molecular formula | C 7 H 4 I 2 O 3 | |||||||||||||||||||||
| Brief description |
light brown powder |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 389.91 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
232–233 ° C ( decomposition ) |
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
3,5-Diiodosalicylic acid is an organic chemical compound that belongs to the group of phenols as well as the group of aromatic carboxylic acids . It is therefore a phenolic acid .
presentation
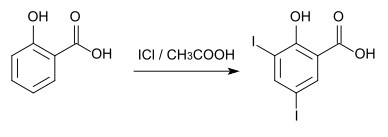
3,5-Diiodosalicylic acid can be prepared from salicylic acid by iodination with iodine chloride in glacial acetic acid .
Furthermore, the synthesis from salicylic acid and elemental iodine in ethanolic solution is possible by adding hydrogen peroxide .
toxicity
In animal experiments it was found that 3,5-diiodosalicylic acid is not toxic.
use
3,5-diiodosalicylic acid is a starting product for the production of rafoxanide , which is used as a drug against tapeworm infestation in pets. In biochemistry , 3,5-diiodosalicylic acid is of interest because, in contrast to unsubstituted salicylic acid, it has a stronger bond to transthyretin .
Reactions
Further iodination of 3,5-diiodosalicylic acid in the presence of potassium hydroxide fails and the acid group 2,4,6-triiodophenol is split off . 3,5-Diiodosalicylic acid turns a ferric chloride solution violet.
Derivatives
Acetylation of 3,5-diiodosalicylic acid leads to diiodo aspirin, the anticoagulant effect of which is greater than that of unsubstituted aspirin . 3,5-diiodo-4- aminosalicylic acid , which is produced by iodination of 4-aminosalicylic acid with iodine chloride in glacial acetic acid, has been used in the treatment of tuberculosis .
Salts
The sodium and ammonium salts of 3,5-diiodosalicylic acid are water-soluble. The salts with rare earth metals have the general formula X (I 2 Sal) 3 · 5 H 2 O, where X = La , Ce , Pr , Nd , or X (I 2 Sal) 3 · 4 H 2 O, where X = Sm , Ho , Yb , Y . These salts are not soluble in water, dimethylformamide , dimethyl sulfoxide and in non-polar solvents, but are soluble in ethanol , methanol and acetone .
The potassium salt of 3,5-diiodosalicylic acid is registered with the CAS number 17274-17-8, the lithium salt under CAS number 42935-32-0.
The separation of the 5-iodosalicylic acid formed as an intermediate product in the synthesis is possible via the barium salt, which in the case of 5-iodosalicylic acid dissolves well in water, whereas 3,5-diiodosalicylate is sparingly soluble.
Individual evidence
- ↑ a b c data sheet 3,5-diiodosalicylic acid from Sigma-Aldrich , accessed on June 18, 2017 ( PDF ).
- ↑ a b c data sheet 3,5-diiodosalicylic acid (PDF) from Merck , accessed on March 6, 2010.
- ^ A b c d Henry Watts: A dictionary of chemistry . 1868, p. 157 ( limited preview in Google Book search).
- ^ GH Woollett and WW Johnson: 2-Hydroxy-3,5-Diiodobenzoic Acid In: Organic Syntheses . 14, 1934, p. 52, doi : 10.15227 / orgsyn.014.0052 ; Coll. Vol. 2, 1943, p. 343 ( PDF ).
- ↑ L. Jurd: The Iodination of aromatic compounds. IV. The Iodination of aromatic Hydrocarbons and Nuclear-substituted phenols , in: Australian Journal of Scientific Research, Series A: Physical Sciences , 1950 , 3 , p. 587. bibcode : 1950AuSRA ... 3..587J .
- ^ S. Mittler, GH Benham: Nutritional availability of iodine from several insoluble compounds , in: J. Nutrition , 1953 , pp. 53-58; (PDF; 296 kB) .
- ↑ alchemchina.com: Pharmaceutical intermediates ( Memento June 20, 2012 in the Internet Archive ), accessed November 19, 2016.
- ↑ Entry on Rafoxanide at chemicalland21.com, accessed on June 18, 2017.
- ↑ L. Gales, MR Almeida, G. Arsequell, G. Valencia, MJ Saraiva, AM Damas: Iodination of salicylic acid improves its binding to transthyretin. In: Biochimica et Biophysica Acta . Volume 1784, Number 3, March 2008, pp. 512-517, doi : 10.1016 / j.bbapap.2007.11.014 , PMID 18155178 .
- ↑ TJ Mende: Enhancement of the antihemostatic effect of acetylsalicylic acid by ring iodination , in: Pharmacology , 1972 , 7 , pp. 249-254; doi : 10.1159 / 000136295 .
- ^ DS Bhate, TB Panse, K. Venkataraman: Antitubercular compounds, Part II, 3,5-Diiodo-4-aminosalicylic acid, 4-Amino-O-acetylsalicylic acid and other derivatives of 4-aminosalicylic acid , 1950 ; 32: 357. doi : 10.1007 / BF03172507 (PDF; 133 kB) .
- ↑ a b c D. K. Koppikar, S. Soundararajan: "Diiodosalicylates of the rare earths", in: Curr. Sci. , 1976 , 45 (1), p. 3; (PDF; 237 kB) .