HCN channel
| HCN channel | ||
|---|---|---|
| Secondary to quaternary structure | Heteromultimer, multi-pass membrane protein | |
| Identifier | ||
| Gene name (s) | HCN1 , HCN2 , HCN3 , HCN4 | |
| Transporter classification | ||
| TCDB | 1.A.1 | |
| designation | Voltage gated ion channels | |
HCN channels (from English : hyperpolarization-activated cyclic nucleotide-gated cation channel ) represent a small subfamily of the protein group of the ion channels . They are a subgroup of the cyclonucleotide-regulated cation channels, which in turn belong to the family of pore loop cation channels.
At present in the human genome four isoforms of HCN channels known (the paralogs HCN1 to HCN4), mainly used in heart and brain expressed are. The current carried by HCN channels is also referred to in many cases as 'pacemaker current', as it is involved in controlling the heart rhythm and promotes rhythmic activity in spontaneously active nerve cells . It is designated either as I h (for hyperpolarization ) or I f (for funny ). The latter name is due to the unusual or strange (= funny ) properties of the channel, such as activation by hyperpolarization instead of the more common depolarization.
Topology of HCN channels
The general structure of an HCN channel complex is always the same: The complex is formed by the juxtaposition of four individual HCN proteins (subunits), which in vivo form four different homotetramers that differ from one another in their biophysical properties. The subunits are attached to one another and form a central pore that allows ions to pass through. Each subunit consists of three important structures: the cytosolic N-terminus , the cytosolic C-terminus with the cyclonucleotide binding domain and the transmembrane domain , which contains the pore and is mechanistically involved in channel opening. (see picture)
Cyclonucleotide binding domain
The cyclic nucleotide binding domain (CNBD ) has a modulating effect on the channel opening, depending on whether cyclic adenosine monophosphate (cAMP) is bound or not. It is connected to the transmembrane domain via the so-called C-linker . There are already crystal structures showing CNBDs with bound cAMP. The pocket in which the cAMP binds has seven amino acid residues that interact with the ligand . These amino acid residues have different roles. The residue R632 mediates the effectiveness of a cAMP binding on the channel opening.
The binding of cAMP to the channel leads to increased channel activity, since the slight change in conformation during binding removes a lasting inhibition (tonic inhibition).
According to one model, the compact C-linker in the absence of cAMP has an inhibitory effect on the channel opening. If cAMP binds to the cyclonucleotide binding domain, this leads to a change in the conformation of the C-terminus. This change results in decreased inhibition and destabilizes the closed state. Consequently, cAMP increases the probability of opening the HCN channels. This shifts the voltage dependency of the activation. This means that at a given cAMP concentration, the channels behave as if they were exposed to a stronger hyperpolarization. Thus there is a "higher pressure" to open.
Transmembrane domain
The transmembrane part of an HCN subunit consists of six alpha-helical segments (S1-S6) and an ion-conducting pore loop between S5 and S6. A highly conserved asparagine residue in the extracellular loop between S5 and the pore loop is glycosylated . This modification is essential for normal surface expression of the HCN channels.
The voltage sensor of the HCN channels is in the S4 segment. It consists of nine arginine or lysine residues , which are located at every third position in the S4 segment. Positively charged S4 segments are found in all voltage-dependent cation channels with a 6 transmembrane domain topology. However, HCN channels form a special feature here. Inward movement of the segment through the plane of the cell membrane in response to hyperpolarization will result in channel opening, while for all other channels it will result in closure. This effect is the subject of current research. Initial evidence suggests the connection of S4 to S5 as a molecular correlate of this different response to tension.
Heteromerization
It could also be shown that the isoforms HCN1 and HCN2 co-assemble with one another and form hetero multimers . The expression of concatamers (repeat sequences of a DNA chain) of HCN1 and HCN2 subunits in mice shows that the kinetics of the channel formed is intermediate between those of the individual isoforms. These results make it clear that the formation of heteromultimers in cells that express both isoforms is possible. What is interesting about the heteromultimers is that each subunit transfers a specific characteristic to the channel. The half-maximal activation was close to that of HCN2 homomultimers, whereas the rate of activation corresponded to that of an HCN1 homomultimer. In fact, the reaction of the HCN1 / HCN2 channels to cAMP was exactly the same as that of the HCN2 channels. A heteromultimerization of other stoichiometry with varying isoforms is accordingly also conceivable in the native tissue. Further experiments also suggest that native HCN channels can contain β subunits.
The co-expression of the protein MinK-related peptide 1 (MIRP1) with HCN1 or HCN2 increased the current amplitude significantly. MIRP1 could either improve surface expression or stabilize the channel complex. In addition, MIRP1 influences the activation and deactivation kinetics of the HCN isoforms, which could explain the variability of the kinetics in various studies. However, the effect of MIRP1 with HCN channels could not be reproduced by all groups. An interaction of this KCNE protein with HCN is therefore unsafe.
Biophysics of the HCN channels
Voltage dependence of activation
The voltage dependency of the activation is different for the different HCN channels. The approximate voltage of half-maximum activation is −70 mV for HCN1 , −95 mV for HCN2, it is in the range of −77 mV to −95 mV for HCN3 and −100 mV for HCN4. In 2005 another voltage-dependent effect could be shown.
Both the HCN channel of the sea urchin sperm (spHCN) and the HCN1 channel of mammals can switch between two different modes, depending on the previous activity. In mode 1 the channel opens at very negative voltages, while in mode 2 this opening is shifted 50 mV into the positive, i.e. it takes place earlier. When open, the channel changes from mode 1 to mode 2, and vice versa when it is closed. This hysteresis effect could also be observed for the HCN2 channel , albeit much weaker than for the HCN1 channel. The effect could not be observed for the HCN4 channel. This mode change leads to a voltage hysteresis, with functional relevance for the activation-dependent "short-term memory" of the HCN channel.
Activation kinetics
The kinetics of activation as well as the voltage dependence are different for the different isoforms. HCN1 is the channel with the fastest opening kinetics with a tau value of 25 to 300 ms depending on the voltage. The HCN4 channel has the slowest opening kinetics with a tau value that ranges from a few hundred milliseconds to several seconds. The kinetics of HCN2 and HCN3 are between those of HCN1 and HCN4.
The voltage dependence and the activation kinetics of the HCN channels are very strongly influenced by experimental parameters (e.g. measurement protocol, temperature) and the intracellular milieu. This property of the HCN channels could explain the variability in biophysical parameters that different groups could observe.
Single channel conductivity
The single-channel conductivity of the HCN channels is controversial. The single-channel conductivity described for the first time, which could be confirmed by more recent measurements, was in the range of 1 pS and was therefore very small. However, individual channel conductivities up to 30 times greater have also been published. The observed difference may be due to the different measurement configurations, but it could also be dynamically changed by in vivo regulation mechanisms. It should also be noted that some of the individual channels did not correspond to the kinetics expected of HCN channels. A final determination of the individual channel conductivity therefore remains to be seen.
Ion selectivity
HCN channels conduct both sodium and potassium ions in a ratio of 1: 4. They are blocked by millimolar concentrations of cesium . A low conductivity for calcium was also described, the functional relevance of which has not yet been clarified.
Although HCN channels also conduct sodium, they carry the GYG motif typical of potassium channels, which forms the selectivity filter. This initially suggested that the channel selectively only lets potassium through. So far it is unclear how the channel conducts both types of ions. However, it is assumed that the GYG motifs of the individual subunits are at a greater distance from one another than in “pure” potassium channels and thus enable the larger sodium to pass through. The extracellular potassium concentration has a great influence on the current amplitude, but also on the ratio of the conductivities for sodium and potassium. An increase in the extracellular potassium concentration leads to an increased current amplitude with a somewhat lower ratio of potassium to sodium conductivity.
In the absence of potassium, HCN channels hardly conduct any sodium. HCN channels do not conduct anions , yet they respond to a change in the extracellular chloride concentration .
Modulation by cAMP
Although all four HCN subunits have highly conserved C-linkers and cyclonucleotide binding sites, the modulation by cAMP is different. The voltage dependency of the activation of HCN2 and HCN4 is shifted by +10 to +25 mV by administration of cAMP. In contrast, HCN1 and HCN3 are only weakly regulated by cAMP. If the cyclonucleotide binding site of HCN4 is replaced by that of HCN3, the cAMP sensitivity is completely retained. The binding site of HCN3 is therefore able to bind cAMP. However, a change in the subunit structure of HCN3 appears to ensure that the cyclonucleotide binding site is functionally disabled.
Expression of HCN channels
The expression of the various HCN channels is largely restricted to heart muscle and nerve tissue . HCN1 is strongly expressed in the brain , whereas HCN2 and HCN4 are more present in the heart. The HCN1 channel is strongly expressed in the brain. There the canal is mainly found in the neocortex , the CA1 region of the hippocampus , in the superior colliculi and in the molecular layer of the cerebellum. A working group was able to demonstrate a robust expression of HCN1 channels in the sinus node and the Purkinje fibers of the rabbit heart, but this heart region is strongly innervated and contamination with neuronal cells cannot be ruled out. In the human sinus node it was recently shown that the HCN1 channel co-expresses with the HCN4 channel.
The HCN2 channel is also found in numerous areas of the brain, such as the olfactory bulb ( olfactory bulb ), the hippocampus , the thalamus and the amygdala . In the heart, the canal can be detected both in the ventricle and in the atrium (atrium). The HCN2 channel is also found in the sinus node in some species such as rats and mice . The HCN3 channel is - in relation to the whole organism - the least expressed isoform. In the nervous tissue it is found very weakly expressed in the olfactory bulb, otherwise HCN3 signals are hardly stronger than the background signal of the in situ hybridizations . In the heart, the channel is only found in very few cells of the conduction system . In addition, the HCN3 channel plays a special role with regard to tissue expression. In addition to nerve and heart muscle tissue, it was the only HCN isoform to be detected in the kidneys and liver . So far there is no data on its function in these tissues. A massive expression of HCN4 can be shown in various thalamic neurons and in the mitral cell region of the olfactory bulb. The HCN4 channel is the dominant isoform in the sinus node in all of the species examined so far. It accounts for about 80% of the HCN expression there. The remaining proportion varies from species to species and is either dominated by HCN1 (in rabbits and humans) or by HCN2 (in mice). HCN4 expression also predominates in other parts of the conduction system ( AV nodes , Purkinje fibers). In mouse heart muscle cells, however, HCN4 plays a less important role, where HCN2 dominates.
Regulation of HCN channels
HCN channels are regulated very intensively by both extra- and intracellular mechanisms. The surface expression, the localization in certain compartments of the cell and the functional properties of the channel are influenced. Regulation can take place both through low molecular weight substances and through interaction with other proteins.
Interaction with low molecular weight substances
Interaction with Ions
It is known that both chloride ions and protons can regulate HCN channels. As already mentioned, HCN channels are pure cation channels. However, their conductivity is influenced by the concentration of free chloride ions. If extracellular chloride is replaced by anions that are larger, the current carried through HCN channels becomes smaller. This sensitivity is mediated by an arginine residue, which is only found in HCN2 and HCN4, but not in HCN1. The effect can therefore only be observed with these isoforms. Regulation by chloride is possibly relevant for physiological processes in the heart.
Protons can regulate HCN channels both intra- and extracellularly. A single histidine residue in the HCN2 of the mouse is responsible for the intracellular reaction to changed pH values. As a result of changes in the pH value, the potential of half-maximal activation shifts to more hyperpolarized values at an acidic pH value, to more depolarized values at an alkaline pH value compared to the physiological pH value of 7.4. Extracellular pH has an impact on HCN1 and HCN4 channels that are found in rat taste cells. A pH value less than 5.0 activates the channels by accelerating the activation kinetics and lowering the activation threshold so that the channels open earlier.
Interaction with phosphatidylinositol 4,5-bisphosphate (PIP2)
PIP2 is an allosteric activator of HCN2 channels that binds to the channel from the intracellular side and facilitates channel activation. This also happens in the absence of cyclic nucleotides. By interacting with PIP2, HCN channels only enter a physiological opening area. In addition, the channel activity can be regulated by the cell by breaking down PIP2 and thus adapted to the needs of the cell.
Interaction with other proteins
Interaction with kinases
An interaction with HCN channels has been described for the protein kinases Src and p38-MAP . The tyrosine kinase Src has been shown to bind to the subunits of HCN1, HCN2 and HCN4. An interaction with the kinase leads to the phosphorylation of a tyrosine residue, which is conserved by the entire HCN family. This phosphorylation accelerates the opening of the channel and the voltage of the half-maximal activation shifts to more positive potentials. The regulation of HCN by Src kinases has already been shown in the heart of mice and rats, as well as in neurons.
Interaction with transmembrane proteins
Like many other ion channels, the HCN channel consists of a transmembrane complex that other transmembrane proteins can attach to in order to interact with the channel. An interaction with various HCN channels has been published for the protein MiRP1. The protein consists of a single transmembrane domain that attaches to the channel. This increases the current density and speeds up the channel opening of HCN2 and slows that of HCN4. How this controversial effect is transferred to the actually very similar subunits is currently not known. Another transmembrane protein that HCN subunits can interact with is KCR1. It reduces the current density of HCN2 and influences the single-channel conductivity. Here, too, the mechanism has not yet been clarified.
Physiological role of the HCN channels
Role of the HCN channels in neurons
In some nerve cells (neurons) in the central nervous system , HCN channels act as real pacemakers. This function has been best studied in switching neurons in the thalamus . Here the HCN channel causes a depolarization followed by an action potential, triggered by T-type calcium channels. In Purkinje cells of the cerebellum , HCN channels are not real pacemakers, but they prevent an excessively long refractory period after an action potential by counteracting hyperpolarization and quickly setting the cell back to the original membrane potential.
It is also assumed that HCN channels are involved in the transmission and integration of exciting (excitatory) synaptic influences. The current of the HCN channels works like a short circuit that prevents an excessively long and strong excitation. If HCN channels are blocked, there is increased temporal summation. It could also be shown that the density of the HCN channels increases with the distance from the nerve cell body ( perikaryon ). This accelerates distal excitations relative to proximal ones and ultimately leads to an identical time course of all excitations of a dendrite .
It was only in 2007 that HCN channels were found to have an influence on the control of spatial memory. By regulating the intracellular cAMP concentration, which is lowered via α-adrenergic receptors and increased via dopamine receptors , the probability of the HCN channels being open is modulated. The membrane resistance in the neurons is adjusted by regulating the HCN channels. This can make it easier or more difficult to respond to incoming information. Unimportant information is filtered through prior activation of the HCN channels and important information is processed more easily in concentration phases. In addition to spatial memory, HCN1 also has a function for learning motor skills. A deletion of HCN1 causes severe learning deficits in this area.
HCN channels also seem to be involved in nicotine dependence. In neurons in the medial habenula of mice, HCN channel-controlled, spontaneous action potentials with a frequency of 2–10 Hz could be detected. Pharmacological blockade of the HCN channels in the medial habenula in vivo led to nicotine withdrawal-like behavior in mice.
Role of the HCN channels in the heart
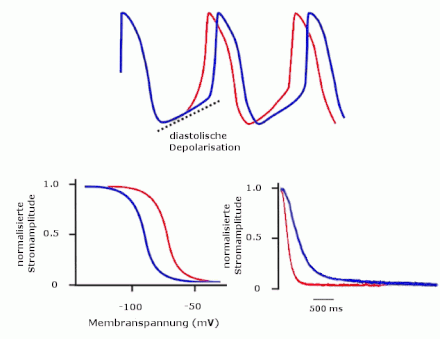
The best-known function of the HCN channels is to generate a cardiac pacemaker potential . Here their current acts as the main component of the diastolic depolarization in the sinus node . The β-adrenergic stimulation of the heart rate also takes place via the effect of cAMP on HCN channels. A sympathetic stimulation activates the channels and thereby accelerates the heartbeat, vagal stimulation slows the heart rhythm.
HCN4 is essential in the development of mouse heart cells. Without an expression of HCN4, their pacemaker cells do not develop into adult cells and the animals die in the womb.
Knockout attempts
Various mouse knockout models were created to investigate the possible functions of the individual HCN channels . These models provide information about the physiological and pathophysiological relevance of the HCN channels.
HCN1 knockout
A global gene knockout of HCN1 in mice leads to a defect that affects the learning of motor tasks. Performing fast movements is particularly affected. This effect is attributed to the loss of HCN1 in the Purkinje neurons of the cerebellum. There is a clear difference in response to membrane hyperpolarization. The HCN1-deficient Purkinje neurons remain in the hyperpolarized state for much longer and reduce their rate of action potential. While the rate of fire in wild-type Purkinje neurons is independent of the initial state, the initial state of excitation has an effect on the rate of fire in HCN1-deficient animals. This influence seems to hinder motor learning. In CA1 neurons, the loss of HCN1 has a significantly different effect. Here he improves spatial memory, both in the short and long term. This effect is mediated by an improved long-term potentiation on CA1 neurons.
HCN2 knockout
Both a neuronal and a cardiac phenotype can be observed in HCN2-deficient animals. Even outwardly, the animals differ from their wild-type conspecifics through reduced activity and ataxia . Absence epilepsy could be demonstrated electrophysiologically in the mutants . The thalamocortical neurons here presumably react excessively strongly to excitations from the cerebral cortex . In addition, the loss of HCN2 channels leads to a hyperpolarized resting membrane potential, which enables the normally inactivated T-type calcium channels to be activated and thus leads to possible oscillations . The cardiac phenotype of HCN2 knockout is sinus arrhythmia , characterized by varying RR intervals with an otherwise normal electrocardiogram . This effect is based on the loss of HCN2 channels in cardiac muscle cells and is not a secondary effect of an HCN2 knockout in nerve cells. The fact that the mice are neither bradycardial nor have exercise problems suggests that the HCN2 channel is not needed for modulating the heart rate . In the sinus node, the current amplitude is reduced by 25%. In addition, the maximum diastolic potential is 5 mV more negative than in the wild type. The lack of stabilization of the resting membrane potential by HCN2 leads to a delay in the following action potential, so that irregularities in the heart rhythm occur.
HCN4 knockout
A global or cardiospecific loss of HCN4 channels leads to the death of the animal in utero between days 10 and 11.5. The heart rate of the animals is 40% lower and no reaction to cAMP can be observed. Loss of the HCN4 channel leads to loss of pacemaker potential. This demonstrates the importance of HCN4 channels in the normal functioning of the heart. If the HCN4 channel is suppressed in adult animals, sinus pauses occur. However, the animals are neither bradycardial nor do they show an incorrect modulation of the heart rhythm.
Therefore, the HCN4 channel appears to be less essential in the adult than in embryos . For non with beta-blockers treatable angina pectoris patients with stable sinus rhythm treatment with can I f channel inhibitors such as ivabradine inhibit the open HCN4 channels and the heart rate lowering.
Pathophysiological role of the HCN channels
HCN channels have physiological relevance as primary and secondary pacemakers, especially in the heart and brain. This is why these organ systems are also pathophysiologically affected by changes in HCN channels. The clinical pictures described so far as a result of HCN mutations are epilepsy , neuralgia and cardiac arrhythmia.
epilepsy
Absence epilepsies in an animal model
A direct effect of changes in HCN channels on epilepsy has currently only been clearly demonstrated in animal models. An example is the model of the rat from the WAG / Rij strain, which serves as a model for epilepsies of the absence type: As early as 1986, spike-wave discharges were detected for the inbred WAG / Rij-Wistar strain , which were accompanied by mild clinical symptoms . These are typical of absence-type epilepsies. They cannot be distinguished from their parent strain Wistar by behavioral studies, so that phenotypically the absence-like seizures stand out as the only differentiating feature. The seizure symptoms are extremely similar to those in humans and only differ in the frequency of the discharges. However, all other symptoms are the same as those of a human seizure. The generation of bilateral (bilateral) synchronous spike-wave discharges is only possible in an anatomically and functionally healthy cortico-thalamic network, which is also in a favorable initial state. This is characterized by a slight hyperpolarization of the pyramidal cells of the cerebral cortex and the thalamic nuclei, which make them susceptible to high-frequency action potential sequences.
A genetic component of absence epilepsy is all but certain in both humans and the rat. This could be shown for the calcium-dependent potassium channel and the T-type calcium channel as well as for the HCN channels HCN2 and HCN1. The importance of HCN1 currents in absence epilepsies is particularly clear in two publications. In the first publication, the flow of thalamocortical neurons in WAG / Rij rats was blocked with cesium or the HCN blocker ZD7288 , which led to an increased discharge of the neurons. Compared to the wild type, a change in the potential of half-maximal activation towards more negative potentials could be shown (−93.2 mV in WAG animals compared to −88.0 mV in the wild type). The WAG neurons responded less well to cAMP. In addition, the expression of the mRNA and the protein of the HCN1 channel was significantly increased compared to the wild type. The group concludes that the increase in expression with the reduced response to cAMP is responsible for the excessive excitation of thalamocortical neurons in WAG animals and thus causally for absence seizures. The second publication describes that a rapid loss of the HCN1 transcript precedes the first attack. This loss is mainly observed in apical dendrites of pyramidal cells of the cerebral cortex. The Ih current carried by HCN1 channels, among other things, is roughly halved. This facilitates somatodendritic communication and declining action potentials can cause calcium influx even at a low frequency. There is an intrinsically high rate of fire in many of these neurons. In addition, after the loss of the HCN1 channel, additional calcium channels are recruited, which are responsible for increased discharges. These mechanisms cause a pathological synchronization of the cortical currents. As already described, synchronous currents can ultimately lead to an epileptic seizure.
HCN and epilepsy in humans
Although rat and mouse models clearly show a pathophysiological role of HCN channels, evidence of a role in human epilepsy has not yet been established. Changes in the expression of HCN channels in humans have so far only been detected in the end-stage of a disease and could therefore also be attributed to regulatory mechanisms. Various circumstances make research into the role of HCN in the various forms of epilepsy difficult. Different animal models for different forms of epilepsy show that both an upregulation and a downregulation of HCN subunits can be associated with epilepsy. The different subunits are involved in the respective diseases to a different extent, depending on their cellular location. Therefore, the role of HCN in epilepsy must always be very differentiated and considered in relation to the specific epilepsy. Research in this direction will reveal further findings in the future.
neuralgia
Various observations suggest that HCN channels play an important role in the development of neuralgia . An expression of HCN1, HCN2 and HCN3 could be detected in the dorsal root ganglia neurons of mice and rats. If these neurons are injured, the current density increases. The cause of the resulting pain could be treated with the HCN channel blocker ZD7288. The mechanism by which the HCN current density is upregulated in the event of an injury is not yet known. Interestingly, the expression of the channels is downregulated in the event of an injury. How this is compatible with the increased current density remains to be investigated.
Cardiac arrhythmia
So far, 4 inherited mutations in HCN channels have been described in the scientific literature. Interestingly, all of these mutations are found in the HCN4 subunit. They lead to sinus bradycardias. So far only heterozygous carriers of traits have been described. This may be due to the serious effects of a mutation on both alleles, which is shown, among other things, in the early lethality of HCN4 knock-out mice. The mutations described are G480R and S672R, which both lead to a shift in the activation curve to more hyperpolarized potentials, 573X, in which a large part of the C-terminus is missing and which can no longer react to a change in the cAMP concentration, and D553N. This mutation leads to a greatly reduced surface expression of the channel. Although HCN4 is also expressed in the central nervous system, there is no neuronal phenotype in these patients.
For the subunits HCN1-3 it is speculated whether a mutation is always lethal in humans, in contrast to the mouse, where the knock-out is tolerated. Alternatively, it is conceivable that mutations in these subunits have not yet been discovered due to their rarity.
HCN channels in medical therapy
cardiology
A heart rate that is too high is positively correlated with the occurrence of diseases such as ischemia and high blood pressure . Due to their great role in the generation of the heart rhythm, HCN channels are of course potential points of attack for causal therapy. A specific active ingredient for HCN channels would also be significantly more tolerable compared to current therapies such as β-blockers , since HCN channels can be specifically addressed in the heart due to their expression profile. Previous therapies mostly suffered from side effects on the blood vessels and the airways. A current development in this direction is the drug ivabradine , which was the first HCN4 blocker ( I f channel inhibitor ) to be approved for therapeutic purposes. The substance specifically blocks HCN4 channels in the sinus node in the low micromolar range ( IC 50 ≈1–2 μM). It settles into the pore of the HCN canal from the inside. This leads to a slowed depolarization of the cells in the sinus node and thus to a lower heart rate. It is currently used as a therapeutic agent for angina pectoris in patients who cannot be treated with beta blockers . In addition to ivabradine, there are a number of other blockers of the HCN flow, for example zatebradine , cilobradine or ZD7288, which is often used experimentally. However, only ivabradine is currently approved for therapy in humans, since the other substances are either not specific enough for cardiac HCN currents, which can lead to unpleasant neuronal side effects, or they block other ion channels in addition to HCN channels.
neurology
As already mentioned in the section on pathophysiology, HCN channels are also involved in the development of neuralgia. It is therefore not surprising that specific blockers can be of great use in the analgesic therapy of these neuralgia. Systemic administration of such a blocker would definitely cause cardiac side effects. To avoid these side effects, subtype-specific blockers for the neuronally expressed subunits HCN1 and HCN2, which do not act on HCN4 channels, would be helpful. These substances do not currently exist, but such a development is not impossible. In addition to the therapy of neuralgia, neuronal HCN agents are also discussed in the context of epilepsy. Here, however, the mechanistic side has not yet been clarified well enough. Depending on the type of epilepsy, either a blockage or an activation of HCN currents would be helpful. This has already been demonstrated in animal models for different forms of epilepsy.
further reading
- M. Biel, C. Wahl-Schott, S. Michalakis, X. Zong: Hyperpolarization-activated cation channels: from genes to function. In: Physiological reviews . Volume 89, Number 3, July 2009, pp. 847-885, ISSN 0031-9333 . doi: 10.1152 / physrev.00029.2008 . PMID 19584315 . (Review).
See also
Individual evidence
- ↑ FH Yu, V. Yarov-Yarovoy, GA Gutman, WA Catterall: Overview of molecular relationships in the voltage-gated ion channel superfamily. In: Pharmacological reviews . Volume 57, Number 4, December 2005, pp. 387-395, ISSN 0031-6997 . doi: 10.1124 / pr.57.4.13 . PMID 16382097 . (Review).
- ^ H. Peter Larson: How is the heart rate regulated in the sinoatrial node? Another piece to the puzzle. In: JGP. 136 (3), 2010, pp. 237-241, doi: 10.1085 / jgp.201010506 . (Full text)
- ^ M. Biel, C. Wahl-Schott, S. Michalakis, X. Zong: Hyperpolarization-activated cation channels: from genes to function. In: Physiological reviews. Volume 89, Number 3, July 2009, pp. 847-885. PMID 19584315 .
- ↑ a b c C. Wahl-Schott, M. Biel: HCN channels: structure, cellular regulation and physiological function. In: Cellular and molecular life sciences: CMLS. Volume 66, Number 3, February 2009, pp. 470-494, ISSN 1420-9071 . doi: 10.1007 / s00018-008-8525-0 . PMID 18953682 . (Review).
- ↑ WN Zagotta, NB Olivier, KD Black, EC Young, R. Olson, E. Gouaux: Structural basis for modulation and agonist specificity of HCN pacemaker channels. In: Nature . Volume 425, Number 6954, September 2003, pp. 200-205, ISSN 1476-4687 . doi: 10.1038 / nature01922 . PMID 12968185 .
- ↑ a b L. Zhou, SA Siegelbaum: Gating of HCN channels by cyclic nucleotides: residue contacts that underlie ligand binding, selectivity, and efficacy. In: Structure (London, England: 1993). Volume 15, Number 6, June 2007, pp. 655-670, ISSN 0969-2126 . doi: 10.1016 / j.str.2007.04.012 . PMID 17562313 . PMC 1995447 (free full text).
- ↑ BJ Wainger, M. DeGennaro, as Santoro, SA Siegelbaum, GR Tibbs: Molecular mechanism of cAMP modulation of HCN pacemaker channels. In: Nature. Volume 411, Number 6839, June 2001, pp. 805-810, ISSN 0028-0836 . doi: 10.1038 / 35081088 . PMID 11459060 .
- ↑ J. Wang, S. Chen, SA Siegelbaum: Regulation of hyperpolarization-activated HCN channel gating and cAMP modulation due to interactions of COOH terminus and core transmembrane regions. In: The Journal of General Physiology . Volume 118, 2001, pp. 237-250. PMID 11524455 . PMC 2229504 (free full text).
- ↑ KB Craven, WN Zagotta: CNG and HCN channels: two peas, one pod. In: Annual review of physiology . Volume 68, 2006, pp. 375-401, ISSN 0066-4278 . doi: 10.1146 / annurev.physiol.68.040104.134728 . PMID 16460277 . (Review).
- ↑ B. Much, C. Wahl-Schott, X. Zong, A. Schneider, L. Baumann, S. Moosmang, A. Ludwig, M. Biel: Role of subunit heteromerization and N-linked glycosylation in the formation of functional hyperpolarization -activated cyclic nucleotide-gated channels. In: The Journal of biological chemistry . Volume 278, Number 44, October 2003, pp. 43781-43786, ISSN 0021-9258 . doi: 10.1074 / jbc.M306958200 . PMID 12928435 .
- ↑ a b J. Chen, JS Mitcheson, M. Lin, MC Sanguinetti: Functional roles of charged residues in the putative voltage sensor of the HCN2 pacemaker channel. In: The Journal of biological chemistry. Volume 275, Number 46, November 2000, pp. 36465-36471, ISSN 0021-9258 . doi: 10.1074 / jbc.M007034200 . PMID 10962006 .
- ↑ R. Männikkö, F. Elinder, HP Larsson: Voltage-sensing mechanism is conserved among ion channels gated by opposite Voltages. In: Nature. Volume 419, Number 6909, October 2002, pp. 837-841, ISSN 0028-0836 . doi: 10.1038 / nature01038 . PMID 12397358 .
- ↑ N. Decher, J. Chen, MC Sanguinetti: Voltage-dependent gating of hyperpolarization-activated, cyclic nucleotide-gated pacemaker channels: molecular coupling between the S4-S5 and C-linkers. In: The Journal of biological chemistry. Volume 279, Number 14, April 2004, pp. 13859-13865, ISSN 0021-9258 . doi: 10.1074 / jbc.M313704200 . PMID 14726518 .
- ↑ DL Prole, G. Yellen: Reversal of HCN channel voltage dependence via bridging of the S4-S5 linker and Post-S6. In: The Journal of general physiology. Volume 128, Number 3, September 2006, pp. 273-282, ISSN 0022-1295 . doi: 10.1085 / jgp.200609590 . PMID 16908727 . PMC 2151568 (free full text).
- ↑ C. Ulens, J. Tytgat: Functional heteromerization of HCN1 and HCN2 pacemaker channels. In: The Journal of biological chemistry. Volume 276, Number 9, March 2001, pp. 6069-6072, ISSN 0021-9258 . doi: 10.1074 / jbc.C000738200 . PMID 11133998 .
- ↑ a b c H. Yu, J. Wu, I. Potapova, RT Wymore, B. Holmes, J. Zuckerman, Z. Pan, H. Wang, W. Shi, RB Robinson, MR El-Maghrabi, W. Benjamin , J. Dixon, D. McKinnon, IS Cohen, R. Wymore: MinK-related peptide 1: A beta subunit for the HCN ion channel subunit family enhances expression and speeds activation. In: Circulation Research Volume 88, Number 12, June 2001, pp. E84-E87, ISSN 1524-4571 . PMID 11420311 .
- ^ A b c C. Altomare, B. Terragni, C. Brioschi, R. Milanesi, C. Pagliuca, C. Viscomi, A. Moroni, M. Baruscotti, D. DiFrancesco: Heteromeric HCN1-HCN4 channels: a comparison with native pacemaker channels from the rabbit sinoatrial node. In: The Journal of Physiology . Volume 549, Pt 2, June 2003, pp. 347-359, ISSN 0022-3751 . doi: 10.1113 / jphysiol.2002.027698 . PMID 12702747 . PMC 2342966 (free full text).
- ↑ a b c J. Stieber, G. Stöckl, S. Herrmann, B. Hassfurth, F. Hofmann: Functional expression of the human HCN3 channel. In: The Journal of biological chemistry. Volume 280, Number 41, October 2005, pp. 34635-34643, ISSN 0021-9258 . doi: 10.1074 / jbc.M502508200 . PMID 16043489 .
- ^ R. Männikkö, S. Pandey, HP Larsson, F. Elinder: Hysteresis in the voltage dependence of HCN channels: conversion between two modes affects pacemaker properties. In: The Journal of general physiology. Volume 125, Number 3, March 2005, pp. 305-326, ISSN 0022-1295 . doi: 10.1085 / jgp.200409130 . PMID 15710913 . PMC 2234019 (free full text).
- ↑ F. Elinder, R. Männikkö, S. Pandey, HP Larsson: Mode shifts in the voltage gating of the mouse and human HCN2 and HCN4 channels. In: The Journal of Physiology. Volume 575, Pt 2 September 2006, pp. 417-431, ISSN 0022-3751 . doi: 10.1113 / jphysiol.2006.110437 . PMID 16777944 . PMC 1819464 (free full text).
- ^ A. Bruening-Wright, HP Larsson: Slow conformational changes of the voltage sensor during the mode shift in hyperpolarization-activated cyclic-nucleotide-gated channels. In: The Journal of neuroscience: the official journal of the Society for Neuroscience. Volume 27, Number 2, January 2007, pp. 270-278, ISSN 1529-2401 . doi: 10.1523 / JNEUROSCI.3801-06.2007 . PMID 17215386 .
- ↑ a b B. Santoro, DT Liu, H. Yao, D. Bartsch, ER Kandel, SA Siegelbaum, GR Tibbs: Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain. In: Cell Volume 93, Number 5, May 1998, pp. 717-729, ISSN 0092-8674 . PMID 9630217 .
- ↑ a b c d A. Ludwig, X. Zong, J. Stieber, R. Hullin, F. Hofmann, M. Biel: Two pacemaker channels from human heart with profoundly different activation kinetics. In: The EMBO journal Volume 18, Number 9, May 1999, pp. 2323-2329, ISSN 0261-4189 . doi: 10.1093 / emboj / 18.9.2323 . PMID 10228147 . PMC 1171315 (free full text).
- ^ B. Santoro, S. Chen, A. Luthi, P. Pavlidis, GP Shumyatsky, GR Tibbs, SA Siegelbaum: Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. In: The Journal of neuroscience: the official journal of the Society for Neuroscience. Volume 20, Number 14, July 2000, pp. 5264-5275, ISSN 0270-6474 . PMID 10884310 .
- ^ D. DiFrancesco: Characterization of single pacemaker channels in cardiac sino-atrial node cells. In: Nature. Volume 324, Number 6096, 1986, pp. 470-473, ISSN 0028-0836 . doi: 10.1038 / 324470a0 . PMID 2431323 .
- ↑ a b G. Michels, F. Er, I. Khan, M. Südkamp, S. Herzig, UC Hoppe: Single-channel properties support a potential contribution of hyperpolarization-activated cyclic nucleotide-gated channels and If to cardiac arrhythmias. In: Circulation. Volume 111, Number 4, February 2005, pp. 399-404, ISSN 1524-4539 . doi: 10.1161 / 01.CIR.0000153799.65783.3A . PMID 15687126 .
- ↑ HC Pape: Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. In: Annual review of physiology. Volume 58, 1996, pp. 299-327, ISSN 0066-4278 . doi: 10.1146 / annurev.ph.58.030196.001503 . PMID 8815797 . (Review).
- ^ X. Yu, KL Duan, CF Shang, HG Yu, Z. Zhou: Calcium influx through hyperpolarization-activated cation channels (I (h) channels) contributes to activity-evoked neuronal secretion. In: Proceedings of the National Academy of Sciences . Volume 101, Number 4, January 2004, pp. 1051-1056, ISSN 0027-8424 . doi: 10.1073 / pnas.0305167101 . PMID 14724293 . PMC 327149 (free full text).
- ↑ X. Yu, XW Chen, P. Zhou, L. Yao, T. Liu, B. Zhang, Y. Li, H. Zheng, LH Zheng, CX Zhang, I. Bruce, JB Ge, SQ Wang, ZA Hu , HG Yu, Z. Zhou: Calcium influx through If channels in rat ventricular myocytes. In: American Journal of Physiology - Cell Physiology. Volume 292, Number 3, March 2007, pp. C1147-C1155, ISSN 0363-6143 . doi: 10.1152 / ajpcell.00598.2005 . PMID 17065201 . PMC 1849975 (free full text).
- ↑ AM Frace, F. Maruoka, A. Noma: External K + increases Na + conductance of the hyperpolarization-activated current in rabbit cardiac pacemaker cells. In: Pflügers Archive - European Journal of Physiology . Volume 421, Number 2-3, June 1992, pp. 97-99, ISSN 0031-6768 . PMID 1326752 .
- ^ LP Wollmuth, B. Hille: Ionic selectivity of Ih channels of rod photoreceptors in tiger salamanders. In: The Journal of general physiology. Volume 100, Number 5, November 1992, pp. 749-765, ISSN 0022-1295 . PMID 1282144 . PMC 2229118 (free full text).
- ^ D. DiFrancesco: A study of the ionic nature of the pace-maker current in calf Purkinje fibers. In: The Journal of Physiology. Volume 314, May 1981, pp. 377-393, ISSN 0022-3751 . PMID 6273534 . PMC 1249440 (free full text).
- ↑ a b A. M. Frace, F. Maruoka, A. Noma: Control of the hyperpolarization-activated cation current by external anions in rabbit sino-atrial node cells. In: The Journal of Physiology. Volume 453, 1992, pp. 307-318, ISSN 0022-3751 . PMID 1281504 . PMC 1175559 (free full text).
- ↑ a b C. Viscomi, C. Altomare, A. Bucchi, E. Camatini, M. Baruscotti, A. Moroni, D. DiFrancesco: C terminus-mediated control of voltage and cAMP gating of hyperpolarization-activated cyclic nucleotide-gated channels . In: The Journal of biological chemistry. Volume 276, Number 32, August 2001, pp. 29930-29934, ISSN 0021-9258 . doi: 10.1074 / jbc.M103971200 . PMID 11397812 .
- ^ A. Ludwig, X. Zong, M. Jeglitsch, F. Hofmann, M. Biel: A family of hyperpolarization-activated mammalian cation channels. In: Nature. Volume 393, Number 6685, June 1998, pp. 587-591, ISSN 0028-0836 . doi: 10.1038 / 31255 . PMID 9634236 .
- ^ A b c d e S. Moosmang, M. Biel, F. Hofmann, A. Ludwig: Differential distribution of four hyperpolarization-activated cation channels in mouse brain. In: Biological chemistry. Volume 380, Numbers 7-8, 1999 Jul-Aug, pp. 975-980, ISSN 1431-6730 . doi: 10.1515 / BC.1999.121 . PMID 10494850 .
- ↑ a b c W. Shi, R. Wymore, H. Yu, J. Wu, RT Wymore, Z. Pan, RB Robinson, JE Dixon, D. McKinnon, IS Cohen: Distribution and prevalence of hyperpolarization-activated cation channel ( HCN) mRNA expression in cardiac tissues. In: Circulation research. Volume 85, Number 1, July 1999, pp. E1-e6, ISSN 1524-4571 . PMID 10400919 .
- ↑ a b NJ Chandler, ID Greener, JO Tellez, S. Inada, H. Musa, P. Molenaar, D. Difrancesco, M. Baruscotti, R. Longhi, RH Anderson, R. Billeter, V. Sharma, DC Sigg, MR Boyett, H. Dobrzynski: Molecular architecture of the human sinus node: insights into the function of the cardiac pacemaker. In: Circulation. Volume 119, Number 12, March 2009, pp. 1562-1575, ISSN 1524-4539 . doi: 10.1161 / CIRCULATIONAHA.108.804369 . PMID 19289639 .
- ↑ T. Notomi, R. Shigemoto: Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain. In: The Journal of comparative neurology. Volume 471, Number 3, April 2004, pp. 241-276, ISSN 0021-9967 . doi: 10.1002 / cne.11039 . PMID 14991560 .
- ↑ a b c A. Ludwig, T. Budde, J. Stieber, S. Moosmang, C. Wahl, K. Holthoff, A. Langebartels, C. Wotjak, T. Munsch, X. Zong, S. Feil, R. Feil, M. Lancel, KR Chien, A. Konnerth, HC Pape, M. Biel, F. Hofmann: Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. In: The EMBO journal. Volume 22, Number 2, January 2003, pp. 216-224, ISSN 0261-4189 . doi: 10.1093 / emboj / cdg032 . PMID 12514127 . PMC 140107 (free full text).
- ↑ C. Wahl-Schott, L. Baumann, X. Zong, M. Biel: An arginine residue in the pore region is a key determinant of chloride dependence in cardiac pacemaker channels. In: J Biol Chem. 280, 2005, pp. 13694-13700.
- ↑ X. Zong, J. Stieber, A. Ludwig, F. Hofmann, M. Biel: A single histidine residue determines the pH sensitivity of the pacemaker channel HCN2. In: J Biol Chem. 276, 2001, pp. 6313-6319.
- ^ A b D. R. Stevens, R. Seifert, B. Bufe, F. Muller, E. Kremmer, R. Gauss, W. Meyerhof, UB Kaupp, B. Lindemann: Hyperpolarization-activated channels HCN1 and HCN4 mediate responses to sour stimuli. In: Nature. 413, 2001, pp. 631-635.
- ↑ J. Qu, A. Barbuti, L. Protas, B. Santoro, IS Cohen, RB Robinson: HCN2 overexpression in newborn and adult ventricular myocytes: distinct effects on gating and excitability. In: Circ Res. 89, 2001, pp. E8-E14.
- ^ RB Robinson, H. Yu, F. Chang, IS Cohen: Developmental change in the voltage-dependence of the pacemaker current, if, in rat ventricle cells. In: Pflügers Arch. 433, 1997, pp. 533-535.
- ↑ JY Wu, IS Cohen: Tyrosine kinase inhibition reduces i (f) in rabbit sinoatrial node myocytes. In: Pflügers Arch. 434, 1997, pp. 509-514.
- ↑ NP Poolos, JB Bullis, MK Roth: Modulation of h-channels in hippocampal pyramidal neurons by p38 mitogenactivated protein kinase. In: J Neurosci. 26, 2006, pp. 7995-8003.
- ↑ B. Santoro, SG Grant, D. Bartsch, ER Kandel: Interactive cloning with the SH3 domain of N-src identifies a new brain specific ion channel protein, with homology to eag and cyclic nucleotide-gated channels. In: Proc Natl Acad Sci USA. 94, 1997, pp. 14815-14820.
- ↑ a b X. Zong, C. Eckert, H. Yuan, C. Wahl-Schott, H. Abicht, L. Fang, R. Li, P. Mistrik, A. Gerstner, B. Much, L. Baumann, S. Michalakis, R. Zeng, Z. Chen, M. Biel: A novel mechanism of modulation of hyperpolarization-activated cyclic nucleotide-gated channels by Src kinase. In: J Biol Chem. 280, 2005, pp. 34224-34232.
- ↑ SS Arinsburg, IS Cohen, HG Yu: constitutively active Src tyrosine kinase changes gating of HCN4 channels through direct binding to the channel protein. In: J Cardiovasc Pharmacol. 47, 2006, pp. 578-586.
- ↑ HG Yu, Z. Lu, Z. Pan, IS Cohen: Tyrosine kinase inhibition differentially regulates heterologously expressed HCN channels. In: Pflügers Arch. 447, 2004, pp. 392-400.
- ↑ N. Decher, F. Bundis, R. Vajna, K. Steinmeyer: KCNE2 modulates current amplitudes and activation kinetics of HCN4: influence of KCNE family members on HCN4 currents. In: Pflügers Arch. 446, 2003, pp. 633-640.
- ↑ J. Qu, Y. Kryukova, IA Potapova, SV Doronin, M. Larsen, G. Krishnamurthy, IS Cohen, RB Robinson: MiRP1 modulates HCN2 channel expression and gating in cardiac myocytes. In: J Biol Chem. 279, 2004, pp. 43497-43502.
- ↑ G. Michels, F. Er, IF Khan, J. Endres-Becker, MC Brandt, N. Gassanov, DC Johns, UC Hoppe: K channel regulator KCR1 suppresses heart rhythm by modulating the pacemaker current I (f). In: PLoS ONE . 3, 2008, p. E1511.
- ^ SR Williams, SR Christensen, GJ Stuart, M. Hausser: Membrane potential bistability is controlled by the hyperpolarization activated current I (H) in rat cerebellar Purkinje neurons in vitro. In: J Physiol. 539, 2002, pp. 469-483.
- ↑ JC Magee: Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. In: J Neurosci. 18, 1998, pp. 7613-7624.
- ↑ JC Magee: Dendritic Ih normalizes temporal summation in hippocampal CA1 neurons. In: Nat Neurosci . 2, 1999, p. 848.
- ↑ M. Wang, BP Ramos, CD Paspalas, Y. Shu, A. Simen, A. Duque, S. Vijayraghavan, A. Brennan, A. Dudley, E. Nou, JA Mazer, DA McCormick, AF Arnsten: Alpha2A- Adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in the prefrontal cortex. In: Cell. 129, 2007, pp. 397-410.
- ↑ MF Nolan, G. Malleret, KH Lee, E. Gibbs, JT Dudman, B. Santoro, D. Yin, RF Thompson, SA Siegelbaum, ER Kandel, A. Morozov: The hyperpolarization-activated HCN1 channel is important for motor learning and neuronal integration by cerebellar Purkinje cells. In: Cell. 115, 2003, pp. 551-564.
- ↑ A. Gorlich, B. Antolin-Fontes, JL Ables, S. Frahm, MA Slimak, JD Dougherty, I. Ibanez-Tallon: Reexposure to nicotine during withdrawal increases the pacemaking activity of cholinergic habenular neurons . In: Proceedings of the National Academy of Sciences . tape 110 , no. 42 , September 30, 2013, p. 17077-17082 , doi : 10.1073 / pnas.1313103110 .
- ↑ D. DiFrancesco, P. Tortora: Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. In: Nature. 351, 1991, pp. 145-147.
- ↑ MF Nolan, G. Malleret, JT Dudman, DL Buhl, B. Santoro, E. Gibbs, S. Vronskaya, G. Buzsáki, SA Siegelbaum, ER Kandel, A. Morozov: A behavioral role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons. In: Cell. Volume 119, Number 5, November 2004, pp. 719-732, ISSN 0092-8674 . doi: 10.1016 / j.cell.2004.11.020 . PMID 15550252 .
- ^ A b S. Herrmann, J. Stieber, A. Ludwig: Pathophysiology of HCN channels. In: Pflüger's archive: European journal of physiology. Volume 454, Number 4, July 2007, pp. 517-522, ISSN 0031-6768 . doi: 10.1007 / s00424-007-0224-4 . PMID 17549513 . (Review).
- ↑ J. Stieber, S. Herrmann, S. Feil, J. Löster, R. Feil, M. Biel, F. Hofmann, A. Ludwig: The hyperpolarization-activated channel HCN4 is required for the generation of pacemaker action potentials in the embryonic heart. In: Proceedings of the National Academy of Sciences . Volume 100, Number 25, December 2003, pp. 15235-15240, ISSN 0027-8424 . doi: 10.1073 / pnas.2434235100 . PMID 14657344 . PMC 299971 (free full text).
- ↑ J. Stieber, K. Wieland, G. Stöckl, A. Ludwig, F. Hofmann: Bradycardic and proarrhythmic properties of sinus node inhibitors. In: Molecular Pharmacology . Volume 69, Number 4, April 2006, pp. 1328-1337, ISSN 0026-895X . doi: 10.1124 / mol.105.020701 . PMID 16387796 .
- ↑ Ernst Mutschler: Mutschler drug effects. Pharmacology, clinical pharmacology, toxicology. 10th edition. Stuttgart 2013.
- ↑ Entry on ivabradine. In: Römpp Online . Georg Thieme Verlag, accessed on September 3, 2013.
- ↑ EL van Luijtelaar, AM Coenen: Two types of electrocortical paroxysms in to inbred strain of rats. In: Neuroscience letters. Volume 70, Number 3, October 1986, pp. 393-397, ISSN 0304-3940 . PMID 3095713 .
- ↑ WH Drinkenburg, EL van Luijtelaar, WJ van Schaijk, AM Coenen: Aberrant transients in the EEG of epileptic rats: a spectral analytical approach. In: Physiology & behavior. Volume 54, Number 4, October 1993, pp. 779-783, ISSN 0031-9384 . PMID 8248357 .
- ↑ D. Gauguier, G. van Luijtelaar, MT Bihoreau, SP Wilder, RF Godfrey, J. Vossen, A. Coenen, RD Cox: Chromosomal mapping of genetic loci controlling absence epilepsy phenotypes in the WAG / Rij rat. In: Epilepsia. Volume 45, Number 8, August 2004, pp. 908-915, ISSN 0013-9580 . doi: 10.1111 / j.0013-9580.2004.13104.x . PMID 15270755 .
- ↑ S. Hirose, A. Mitsudome, M. Okada, S. Kaneko: Genetics of idiopathic epilepsies. In: Epilepsia. Volume 46 Suppl 1, 2005, pp. 38-43, ISSN 0013-9580 . doi: 10.1111 / j.0013-9580.2005.461011.x . PMID 15816978 . (Review).
- ↑ G. Gandolfo, S. Romettino, C. Gottesmann, G. van Luijtelaar, A. Coenen, JN Bidard, M. Lazdunski: K + channel openers decrease seizures in genetically epileptic rats. In: European journal of pharmacology . Volume 167, Number 1, August 1989, pp. 181-183, ISSN 0014-2999 . PMID 2506066 .
- ↑ E. Tsakiridou, L. Bertollini, M. de Curtis, G. Avanzini, HC Pape: Selective increase in T-type calcium conductance of reticular thalamic neurons in a rat model of absence epilepsy. In: The Journal of neuroscience: the official journal of the Society for Neuroscience. Volume 15, Number 4, April 1995, pp. 3110-3117, ISSN 0270-6474 . PMID 7722649 .
- ↑ T. Budde, L. Caputi, T. Kanyshkova, R. Staak, C. Abrahamczik, T. Munsch, HC Pape: Impaired regulation of thalamic pacemaker channels through an imbalance of subunit expression in absence epilepsy. In: The Journal of neuroscience: the official journal of the Society for Neuroscience. Volume 25, Number 43, October 2005, pp. 9871-9882, ISSN 1529-2401 . doi: 10.1523 / JNEUROSCI.2590-05.2005 . PMID 16251434 .
- ↑ MH Kole, AU Bräuer, GJ Stuart: Inherited cortical HCN1 channel loss amplifies dendritic calcium electrogenesis and burst firing in a rat absence epilepsy model. In: The Journal of Physiology. Volume 578, Pt 2 January 2007, pp. 507-525, ISSN 0022-3751 . doi: 10.1113 / jphysiol.2006.122028 . PMID 17095562 . PMC 2075144 (free full text).
- ↑ a b S. R. Chaplan, HQ Guo, DH Lee, L. Luo, C. Liu, C. Kuei, AA Velumian, MP Butler, SM Brown, AE Dubin: Neuronal hyperpolarization-activated pacemaker channels drive neuropathic pain. In: J Neurosci. 23, 2003, pp. 1169-1178.
- ↑ J. Kitagawa, M. Takeda, I. Suzuki, J. Kadoi, Y. Tsuboi, K. Honda, S. Matsumoto, H. Nakagawa, A. Tanabe, K. Iwata: Mechanisms involved in modulation of trigeminal primary afferent activity in rats with peripheral mononeuropathy. In: Eur J Neurosci. 24, 2006, pp. 1976-1986.
- ^ H. Yao, DF Donnelly, C. Ma, RH LaMotte: Upregulation of the hyperpolarization-activated cation current after chronic compression of the dorsal root ganglion. In: J Neurosci. 23, 2003, pp. 2069-2074.
- ↑ DH Lee, L. Chang, LS Sorkin, SR Chaplan: Hyperpolarization-activated, cation-nonselective, cyclic nucleotidemodulated channel blockade alleviates mechanical allodynia and suppresses ectopic discharge in spinal nerve ligated rats. In: J Pain. 6, 2005, pp. 417-424.
- ^ Q. Sun, GG Xing, HY Tu, JS Han, Y. Wan: Inhibition of hyperpolarization-activated current by ZD7288 suppresses ectopic discharges of injured dorsal root ganglion neurons in a rat model of neuropathic pain. In: Brain Res. 1032, 2005, pp. 63-69.
- ↑ E. Nof, D. Luria, D. Brass, D. Marek, H. Lahat, H. Reznik-Wolf, E. Pras, N. Dascal, M. Eldar, M. Glikson: Point mutation in the HCN4 cardiac ion channel pore affecting synthesis, trafficking, and functional expression is associated with familial asymptomatic sinus bradycardia. In: Circulation. 116, 2007, pp. 463-470.
- ↑ R. Milanesi, M. Baruscotti, T. Gnecchi-Ruscone, D. DiFrancesco: Familial sinus bradycardia associated with a mutation in the cardiac pacemaker channel. In: N Engl J Med. 354, 2006, pp. 151-157.
- ↑ E. Schulze-Bahr, A. Neu, P. Friederich, UB Kaupp, G. Breithardt, O. Pongs, D. Isbrandt: Pacemaker channel dysfunction in a patient with sinus node disease. In: J Clin Invest. 111, 2003, pp. 1537-1545.
- ↑ K. Ueda, K. Nakamura, T. Hayashi, N. Inagaki, M. Takahashi, T. Arimura, H. Morita, Y. Higashiuesato, Y. Hirano, M. Yasunami, S. Takishita, A. Yamashina, T. Ohe, M. Sunamori, M. Hiraoka, A. Kimura: Functional characterization of a trafficking-defective HCN4 mutation, D553N, associated with cardiac arrhythmia. In: J Biol Chem. 279, 2004, pp. 27194-27198.
- ↑ S. Sulfi AD Timmis: Ivabradine: the first selective sinus node I (f) channel inhibitor in the treatment of stable angina. In: Int J Clin Pract. 60, 2006, pp. 222-228.
- ↑ L. Cervetto, GC Demontis, C. Gargini: Cellular mechanisms underlying the pharmacological induction of phosphenes. In: Br J Pharmacol . 150, 2007, pp. 383-390.
- ↑ M. Kitayama, H. Miyata, M. Yano, N. Saito, Y. Matsuda, T. Yamauchi, S. Kogure: Ih blockers have a potential of antiepileptic effects. In: Epilepsia. 44, 2003, pp. 20-24.
- ^ R. Surges, TM Freiman, TJ Feuerstein: Gabapentin increases the hyperpolarization-activated cation current Ih in rat CA1 pyramidal cells. In: Epilepsia. 44, 2003, pp. 150-156.