Lactose intolerance
| Classification according to ICD-10 | |
|---|---|
| E73 | Lactose intolerance |
| ICD-10 online (WHO version 2019) | |
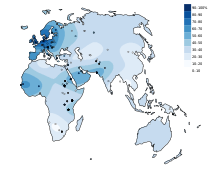
With lactose intolerance , lactose intolerance or lactose intolerance of ingested is milk sugar as a result of missing or reduced production of digestive enzyme lactase not digested or incomplete; this can result in an intolerance to milk and milk products. Lactose intolerance is very rare in children under the age of five. Usually it only develops in adolescence and adulthood. Lactose intolerance is very common worldwide, although there are significant differences depending on the region and population. Around 5 to 15% of Europeans cannot tolerate lactose. Lactose intolerance is rarest in Northern Europe. In Africa or East Asia, on the other hand, 65 to over 90% of adults are affected. The evolutionary advantage of the Northern Europeans, known as lactase persistence , arose from a gene mutation about 7000 years ago in the course of the increasingly important dairy farming.
General
All (healthy) newborn mammals form the enzyme lactase during suckling ( infants during breastfeeding ) , which splits the unabsorbed disaccharide lactose into the usable simple sugars D - galactose and D - glucose . For this purpose, lactase is formed in the thin intestinal mucosa (especially in the jejunum ) and is a component of intestinal juice ; it enables the newborn to use the lactose-rich breast milk as the main source of energy.
In the course of natural weaning from breast milk, lactase activity drops to around 5–10% of the activity at birth. This applies to humans and all other mammals. Only in populations that have been dairy farmers for a long time has a mutation prevailed which means that sufficient lactase is still produced in adulthood; this is known as lactase persistence. This is probably due to the fact that the higher lactase activity offered a selection advantage (minerals, nutritional value) for these groups.
In the case of insufficient lactase activity, unsplit lactose reaches the large intestine in humans, where it is absorbed and fermented by intestinal bacteria . Lactate (the salt of lactic acid ) and the gases carbon dioxide (CO 2 ), methane (CH 4 ) and hydrogen (H 2 ) are usually formed as fermentation products shortly after consumption . The gases lead, among other things, to a feeling of fullness, pelvic pain, flatulence and the short-chain fatty acids which are also formed, to nausea and vomiting. The osmotically active lactic acid causes water to flow into the intestine with diarrhea (osmotic diarrhea ). In Asia and Africa, the lack of lactase persistence affects most of the adult population (90% or more), in Western Europe, Australia and North America it is 5–15% (in fair-skinned people).
Similar symptoms occur in people who are intolerant to fructose , known as fructose malabsorption ; There are also similarities with irritable bowel syndrome .
causes
Lactose intolerance can have several causes:
- Congenital lactase deficiency (absolute lactose intolerance ): Due to a genetic defect , lactase production is severely restricted or no enzyme can be produced at all (so-called alactasia ). Inheritance is autosomal - recessive . It is a rare hereditary disease that can be identified by diarrhea in the first few days after birth.
- Some infants diagnosed with congenital lactose intolerance may have a rare disorder in which undigested lactose is absorbed by the stomach, enters the bloodstream ( lactosemia ) and is excreted in the urine ( lactosuria ). Since undigested lactose is harmful in the blood, the disorder can lead to symptoms of intoxication with cataracts and liver and brain damage undetected .
- Primary (natural) lactase deficiency : This digestive enzyme is usually produced in sufficient quantities in infants. After a few years, however, the amount of lactase produced decreases differently depending on the population. For example, a large part of the adult Central and South Asian population no longer tolerates dairy products , in northern areas (for most of the inhabitants of Europe and the Middle East or people of European and Middle Eastern descent and the Siberian and Mongolian ethnic groups), lactose intake usually continues well into old age no problem. The reason for the drying up of enzyme production in adulthood is a gene on the long arm of chromosome 2 (2q21) in the promoter area of the lactase gene: CC genotype of the C / T-13910 polymorphism or GG genotype of G / A-22018- Polymorphism.
-
Secondary (acquired or temporary) lactose intolerance (according to Leiß 2005), e.g. B. by the following causes:
- Diseases of the digestive system can damage so that the temporary lactase production is impaired, especially during childhood, lactase-producing cells in the small intestine; after curing, this deficiency usually disappears completely. In rare cases, the lactase-producing tissues are so damaged that they can no longer recover, leading to chronic lactose intolerance.
- bacterial or viral gastroenteritis
- chronic bowel disease
- Celiac disease / sprue
- intestinal lymphoma
- partial or total gastrectomy
- Short bowel syndrome
- Blind sac syndrome / large duodenal diverticulum
- Chemotherapy / radiation therapy
- Malnutrition
- chronic alcohol abuse
- Small intestinal parasites from the Giardia group (such as Giardia intestinalis )
diagnosis
A self-diagnosis of lactose intolerance can be carried out with a test sequence:
- Diet test: A consistent diet for several days without lactose, especially without milk, cream and "hidden" lactose (many finished products contain milk sugar or milk components). If there are no more symptoms during this time, lactose intolerance is possible.
- Exposure test: After a few days without lactose, a glass of water with 50 to 100 g of dissolved milk sugar is drunk. If the typical symptoms appear within a few hours, you are probably lactose intolerant.
The diagnosis is often ambiguous because there is only incomplete intolerance. In the usual form, the intolerance increases in the course of life, not so with the congenital lactase deficiency.
The following tests are more complex:
- H 2 breath test : This method is based on the detection of hydrogen (H 2 ) in the exhaled air. It is an indirect proof of lactase deficiency. In addition to lactic acid , acetic acid and carbon dioxide ,the bacterial processing of lactose in the large intestine also producesgaseous hydrogen . This reaches the lungs via the blood and is exhaled. Since there is normally no hydrogen in the exhaled air, a positive result indicates a possible lactose intolerance. This test measures the hydrogen concentration before and after the oral administration of a defined amount of lactose (milk sugar). The result is considered positive if the measurement result before and after the lactose administration shows a difference of 20 ppm hydrogen. However, this test leads to a negative result for every fifth lactose intolerant: These patients have certain (harmless) bacteria in their intestinal florathat produce methane , which means that it is not possible to detect hydrogen.
- Blood sugar test: This procedure is based on the measurement of the glucose content in the blood ( venous blood or capillary blood ), the lactase activity is determined by an increase in the concentration of glucose in the blood. Since lactose is normally broken down into galactose and glucose , the glucose value ( blood sugar value ) should rise if lactose is ingested. If this is not the case, a lactose intolerance is suspected. In this test, too, the patient consumes a defined amount of lactose (usually 50 grams dissolved in half a liter of still water) on an empty stomach. A blood sample is taken before ingestion and every 30 minutes after ingestion for two hours and the blood sugar level is measured. An increase of more than 20 mg / dl (1.11 mmol / l) glucose in venous blood or 25 mg / dl in capillary blood is normal. An increase of less than 10 mg / dl in venous blood is pathological. False negative results are possible in patients with latent or manifest diabetes mellitus .
- Genetic test: A genetic test for the LCT genotype has recently been carried out if lactose intolerance is suspected . A simple blood sample is used as the test material.
- Biopsy: In rare cases, a tissue sample must be taken from the small intestine and examined. Here the lactase activity in the small intestinal tissue is examined.
Lactose malodigestion does not necessarily lead to lactose intolerance
Clinical studies have shown that the primary lactase deficiency (non-persistence) and the resulting digestive weakness for milk sugar (lactose malodigestion) do not necessarily lead to the known symptoms of lactose intolerance. Most people with primary lactase deficiency can tolerate up to 12 g of lactose without symptoms, which is roughly equivalent to a glass of milk (200 ml). Of lactase deficiency test persons with significantly increased breath hydrogen after ingesting 25 g of lactose, about half tolerated this amount without symptoms. There are also people who digest lactose well but still show symptoms of intolerance, as well as people with a lactase deficiency whose symptoms persist even with lactose-free milk. Although the activity of intestinal lactase is not increased by continuously ingesting milk sugar, it can reduce both the breath hydrogen and the gastrointestinal symptoms. Adjustments in colon functions ( motility , transit and pH value) and an associated reduced perception of symptoms, also as a result of an altered metabolism of the colon flora , offer a likely explanation. Various probiotic, but above all live, conventional yoghurt cultures support lactose digestion and reduce the occurrence of gastrointestinal symptoms.
treatment
The effects of the lactase deficiency can e.g. B. can be reduced to a minimum by changing the diet to low or lactose-free foods. There are alternatives in the form of various milk substitute drinks , some of which are also enriched with additional vitamins and calcium . In addition to soy milk , grain or almond milk is available. There are also lactose-reduced dairy products, including milk, but also cheese, yoghurt, cream, quark and more. Another possibility is to supply the enzyme lactase from the outside in the form of chewable tablets or capsules through appropriate pharmaceutical products from the drugstore or pharmacy. However, the effectiveness of these supplements has not been proven beyond doubt.
Milk sugar, on the other hand, is added to many food products, such as breads, cereal bars, ready meals, seasonings, sausages, marinated meats, doughs, candies, ice cream, chocolate and instant products such as sachet soups. One reason for this is the “mouthfeel” desired by the food designer , which has a positive effect on the taste. Most of those affected, however, can tolerate smaller amounts of lactose with almost no symptoms, so that it is not necessary to avoid it completely. Since November 25, 2005, new regulations for the labeling of allergenic food ingredients have been in effect ( Federal Law Gazette I p. 2896 ). The labeling requirement also includes milk and milk components including lactose. Also, functional food and drugs , including birth control pills may contain lactose as an excipient, but in an absolutely negligible quantity.
Fermented milk products, including all sour milk products , cheese and quark, contain z. T. the enzyme lactase by nature, so that only very small amounts of milk sugar remain. This is mainly related to the production process, in particular the amount of bacteria added to the milk, which produce the enzyme lactase and thereby break down the milk sugar, as well as the maturation process and the duration of cheeses and yoghurts. In the case of cheese, the following generally applies: the longer the ripening process, the lower the lactose content.
It often happens that those affected strictly avoid milk and dairy products as a result. Whether or not there is a critical level of calcium deficiency depends on the balance of the other food. Some sources report that lactose intolerants are exposed to an increased risk of osteoporosis . The reason given is the lower consumption of foods containing calcium. With an overall balanced diet, a calcium deficiency is hardly to be expected.
Anthropological Findings
From a 2007 study of the Mainz anthropologist Joachim Burger shows that lactose intolerance adult people a phylogenetically 's original property of the people that that is the ability to digest, even as an adult lactose problems, a relatively recent genetic innovation is. Together with British colleagues, Burger had examined nine European skeletons from the Neolithic and Mesolithic (7800 to 7200 years old) and, while analyzing their genes, discovered that none of these individuals was able to digest milk. On the other hand , a 1500 year old skeleton from the Merovingian period analyzed for control has the genetic modification so that this individual was able to digest lactose. A DNA analysis of the glacier mummy known as Ötzi also revealed that the man, who died around 5000 years ago, was lactose intolerant. The ability of adults to digest milk in Europe only spread in parallel with the expansion of agriculture and after the introduction of animal breeding, which has been taking place here for around 8,000 years. In the genome analysis of 18 skeletons from a medieval cemetery in Dalheim , published in 2013, 13 of them (72 percent) had the genotype for lactase persistence, which corresponds to today's level in Germany and Austria. In October 2014 a study of the prehistoric inhabitants of the Pannonian Plain was published ; with the result that the adjustment process took many thousands of years.
Worldwide
| Region or ethnicity | Lactose intolerance% |
|---|---|
| South East Asia | 98 |
| China | 94 |
| Aboriginal | 85 |
| Inuit (Alaska) | 80 |
| Central Asia | 80 |
| African American | 79 |
| South America | 65-75 |
| Sicily | 71 |
| South india | 70 |
| South of france | 65 |
| Maasai | 62 |
| Crete | 56 |
| Balkans | 55 |
| Southern Italy | 52 |
| Indians | 50 |
| Northern Italy | 41 |
| North India | 27 |
| Bedouin | 25th |
| Tutsi ( Rwanda ) | 20th |
| Central Italy | 19th |
| Finland | 18th |
| Northern France | 17th |
| Switzerland | 15-20 |
| Germany | 15th |
| Tuareg | 13 |
| Whites in the United States | 12 |
| Austria | 10-15 |
| Great Britain | 5-15 |
| Denmark | 5 |
| Sweden | 2 |
Swell:
See also
literature
- Fritz Höffeler: History and evolution of lactose (in) tolerance. In: Biology in Our Time . Volume 39, No. 6, 2009, pp. 378-387. doi : 10.1002 / biuz.200910405 .
- CJ Ingram, CA Mulcare et al. a .: Lactose digestion and the evolutionary genetics of lactase persistence. In: Human genetics. Volume 124, Number 6, January 2009, pp. 579-591, doi: 10.1007 / s00439-008-0593-6 . PMID 19034520 .
Web links
- Lactose intolerance - information at Gesundheitsinformation.de (online offer of the Institute for Quality and Efficiency in Health Care )
- Lactose: Occurrence, properties, metabolism and dietetics - information from the Bavarian State Ministry for the Environment and Consumer Protection
- ZEIT article: Rumbling in the intestines (2007)
- Andrew Curry: The Milk Revolution - A single mutation long ago made milk palatable to Europeans. , at Spektrum.de, last accessed May 3, 2018
Individual evidence
- ↑ a b c Lactose intolerance on informedhealth.org, accessed on April 24, 2020.
- ↑ Yuval Itan et al .: The Origins of Lactase Persistence in Europe. In: PLoS Computational Biology. Volume 5, No. 8, 2009, e1000491, doi: 10.1371 / journal.pcbi.1000491 .
- ↑ Andrew Curry: The Milk Revolution. On: Spektrum.de from August 12, 2013, accessed on April 24, 2020.
- ↑ Oliver Rick: Clinic Guide Medical Rehabilitation. Elsevier, Urban & Fischer 2013, ISBN 978-3-437-59347-5 , p. 31 ( limited preview in the Google book search).
- ↑ Jeremy M. Berg, Lubert Stryer, John L. Tymoczko: Biochemistry. 5th edition. Freeman, 2002, ISBN 0-7167-4684-0 , ch. 16.1.12. (English)
- ↑ A. Beja-Pereira, G. Luikart u. a .: Gene-culture coevolution between cattle milk protein genes and human lactase genes. In: Nature genetics. Volume 35, Number 4, December 2003, pp. 311-313, doi: 10.1038 / ng1263 . PMID 14634648 . (Review).
- ↑ Jeremy M. Berg, Lubert Stryer, John L. Tymoczko: Biochemistry. 6th edition. Spektrum Akademischer Verlag, 2007, ISBN 978-3-8274-1800-5 , p. 504.
- ↑ AC Bulhoes et al. a .: Correlation between lactose absorption and the C / T-13910 and G / A-22018 mutations of the lactase-phlorizin hydrolase (LCT) gene in adult-type hypolactasia. In: Brazilian Journal of Medical and Biological Research. November 2007, accessed July 19, 2008 .
- ↑ Alactasia. In: Online Mendelian Inheritance in Man . (English)
- ^ NO Berg, A. Dahlqvist u. a .: Severe familial lactose intolerance – a gastrogen disorder? In: Acta paediatrica Scandinavica. Volume 58, Number 5, September 1969, pp. 525-527, ISSN 0001-656X . PMID 5365173 .
- ^ G. Russo, F. Mollica et al. a .: Congenital lactose intolerance of gastrogen origin associated with cataracts. In: Acta paediatrica Scandinavica. Volume 63, Number 3, May 1974, pp. 457-460, ISSN 0001-656X . PMID 4209121
- ↑ Y. Hirashima, S. Shinozuka et al. a .: Lactose intolerance associated with cataracts. In: European Journal of Pediatrics. Volume 130, Number 1, January 1979, pp. 41-45, ISSN 0340-6199 . PMID 759181 .
- ↑ A. Hosková, J. Sabacký u. a .: Severe lactose intolerance with lactosuria and vomiting. In: Archives of Disease in Childhood. Volume 55, Number 4, April 1980, pp. 304-305, ISSN 1468-2044 . PMID 7416780 . PMC 1626838 (free full text)
- ↑ I. Jarvela, p Torniainen, KL Kolho: Molecular genetics of human lactase deficiencies . In: Ann. Med. July 2009, p. 1-8 , doi : 10.1080 / 07853890903121033 , PMID 19639477 .
- ↑ O. Leiß: Dietary therapy for carbohydrate malabsorption and lactose intolerance. In: Current. Nourish. Med. Volume 30, 2006, pp. 75-87.
- ↑ Thomas Löscher, Gerd-Dieter Burchard (Ed.): Tropical medicine in clinic and practice . 4th, revised edition. Georg Thieme Verlag, Stuttgart 2010, ISBN 978-3-13-785804-1 , p. 652 ( Google Books ).
- ^ MD Levitt: Production and excretion of hydrogen gas in man . In: The New England Journal of Medicine . 281, No. 5, 1969, pp. 122-127.
- ↑ Operating instructions for the Gastrolyzer H2 breath test device from Specialmed / Bedfont (PDF)
- ↑ J. Futh: Molecular genetic and clinical studies on lactose intolerance (hypolactasia) , dissertation at the Medical Faculty of the University of Rostock, 2008. https://d-nb.info/1000953769/34
- ↑ a b c d e M. de Vrese et al .: Probiotics — compensation for lactase insufficiency , in: American Journal of Clinical Nutrition No. 73 (suppl.) Pp. 421-429, 2001. https://doi.org /10.1093/ajcn/73.2.421s
- ↑ a b R. A. Forsgard: Lactose digestion in humans: intestinal lactase appears to be constitutive whereas the colonic microbiome is adaptable in: American Journal of Clinical Nutrition No. 110, pp. 273-279, 2019. doi: 10.1093 / ajcn / nqz104
- ↑ AO Johnson et al .: Adaptation of lactose maldigesters to continued milk intakes , in: American Journal of Clinical Nutrition No. 58, pp. 879-881, 1993. https://doi.org/10.1093/ajcn/58.6.879
- ^ Lactose intolerance Treatment . Retrieved August 11, 2017.
- ↑ a b c Tuula H. Vesa u. a .: Lactose intolerance. In: Journal of the American College of Nutrition. Vol. 19, No. 90002, 2000, pp. 165S-175S, PMID 10759141 .
- ↑ J. Burger, M. Kirchner, B. Bramanti, W. Haak, MG Thomas: Absence of the lactase-persistence-associated allele in early Neolithic Europeans. In: PNAS . Volume 104, No. 10, of March 6, 2007, pp. 3736-3741, doi: 10.1073 / pnas.0607187104 .
- ↑ Andreas Keller u. a .: New insights into the Tyrolean Iceman's origin and phenotype as inferred by whole-genome sequencing. In: Nature Communications. Volume 3, Article No. 698, 2012, doi: 10.1038 / ncomms1701
- ↑ MI Hofmann, T. Böni, KW Alt, U. Woitek, FJ Rühli: Paleopathologies of the vertebral column in medieval skeletons. In: Anthropologischer Anzeiger ; Report on the biological-anthropological literature. Volume 66, Number 1, March 2008, pp. 1-17, PMID 18435203 .
- ↑ Europeans were lactose tolerant 1,000 years ago. on: derstandard.at , January 29, 2014, accessed on January 29, 2014.
- ↑ Annina Krüttli, Abigail Bouwman u. a .: Ancient DNA Analysis Reveals High Frequency of European Lactase Persistence Allele (T-13910) in Medieval Central Europe. In: PLoS ONE. 9, 2014, p. E86251, doi: 10.1371 / journal.pone.0086251 .
- ↑ Compatibility with lactose came up surprisingly late. @ Spektrum.de with reference to Genome flux and stasis in a five millennium transect of European prehistory. @ nature.com, publ. October 21, 2014; s. a. Ancient DNA from prehistoric inhabitants of Hungary. @ dienekes.blogspot.de, all accessed on October 31, 2014.
- ^ The Molecular Explanation. Information - Concepts In Nutrigenomics - Lactose Intolerance, 2012, The NCMHD Center of Excellence for Nutritional Genomics
- ↑ Lactose Intolerance. In: Kenneth F. Kiple (Ed.): The Cambridge World History of Food. Cambridge 2000, p. 1060.
- ↑ Norman Kretchmer: Lactose and Lactase. In: Scientific American. Oct. 1972 Oct; 227 (4), pp. 71-78.
- ↑ RT Jackson et al. a .: Lactose malabsorption among Masai children of East Africa. In: American Journal of Clinical Nutrition. 1979, Vol. 32, pp. 779-782.
- ^ Lactose intolerance Austrian Nutrition Society, accessed on April 17, 2017.
- ↑ Keller et al. 2012. Sixth Swiss Nutrition Report . Bern: Federal Office of Public Health. Page 163 ( online )