Celiac disease
| Classification according to ICD-10 | |
|---|---|
| K90.0 | Celiac disease |
| ICD-10 online (WHO version 2019) | |
The Celiac Disease ( Synonyms : gluten sensitive or gluten sensitive enteropathy , (rare) intestinal infantilism ; in adults also: not tropical or tropical sprue , Heubner-Herter's disease ) is a by gluten intolerance caused disease of the gastrointestinal tract , both characteristics of an allergy as also has an autoimmune disease . It is especially by a chronic inflammation of the small intestine mucosa due to a hypersensitivity to any component of gluten , which mainly in grains (seeds) of many grains occurring gluten protein characterized. Other organs can also be affected. The intolerance remains lifelong, it is partly hereditary and cannot currently be treated causally.
Foods containing gluten cause inflammation of the thin intestinal mucosa , often with extensive destruction of the intestinal epithelial cells . As a result, nutrients can only be absorbed poorly, they largely remain undigested in the intestine. The symptoms and the severity of the clinical picture can be very different, which makes it difficult to recognize. Possible symptoms are weight loss , diarrhea , vomiting , loss of appetite , tiredness, depression, a tendency to bleed (due to vitamin K deficiency) and failure to thrive in childhood (slow physical development); neurological disorders can also occur. A non therapied celiac disease increases the risk of non-Hodgkin's lymphoma (a lymph node - cancer ) and probably also of cancers of the digestive tract. Celiac disease is associated with type 1 diabetes mellitus in five to ten percent of patients . The treatment of celiac disease currently consists exclusively of a gluten-free diet.
Symptoms similar to those of celiac disease occur with a wheat allergy . As a diagnosis of exclusion “in the case of a wheat-dependent clinic and negative serology (for celiac disease-specific antibodies), normal small intestine histology, negative specific IgE (wheat) and negative prick test (wheat), after carefully excluding other diagnoses, the suspicion of non-celiac disease-not -Wheat allergy-wheat sensitivity . "
Origin of name
Celiac disease is the German equivalent of the neo-Latin technical term coeliacia . The word is derived from the Latin adjective celiacus , with the basic meaning 'relating to the abdomen', but which is also translated as 'lower abdomen' (purely Latin ventriculosus ). The Latin celiacus goes back to the Greek adjective κοιλιακός koiliakós , German 'suffering from digestion' , which is derived from κοιλία koilía , German 'abdominal cavity' , 'belly', 'lower body'.
Allergens
The allergens are the gliadins (the alcohol-soluble fraction of gluten ) and glutenins. Depending on the type of grain, these allergens have a different composition due to the evolution of the types of grain.
- Wheat , spelled (including green spelled ), khorasan , durum : α- / β / ω- gliadin and glutenins , CC allergen (baker's asthma ), CBP2
- Barley : Hordenine amylase inhibitors IAM1 and CMb (both baker's asthma)
- Oats : Avenin A, E and F
- Rye : secalinin
Gluten-free cereals
- Corn
- rice
- Millet (also: teff , brown millet and peeled "gold millet")
All pseudograins are also gluten-free:
Since different types of grain are often processed in the same company, cross-contamination cannot be ruled out. Gluten-free goods are signaled e.g. B. the gf logo (crossed-out ear of wheat), otherwise usually the warning “may contain traces of gluten” (see section EU regulation ).
Epidemiology
The incidence of the disease varies considerably in different countries. In addition, the frequency information differs depending on whether the diagnosis is based on clinical symptoms or based on a screening test in the serum. If only symptomatic cases are taken into account, the frequency ( prevalence ) ranges from 1: 10,000 (Denmark, USA) to 1: 300 (Sweden, Great Britain). Worldwide an average frequency of about 1: 3350 is given. If one also includes the cases diagnosed by screening examinations, the prevalence increases to 1: 500 in Germany and Denmark and about 1: 110 in the USA and Great Britain, the world average about 1: 270. The increasing number of illnesses in Sweden while the incidence remains constant in the genetically related northern Denmark is attributed to the early feeding of grain-based complementary foods in infancy, which is common in Sweden . Feeding too early seems to increase the risk of developing celiac disease. In people with Down syndrome (trisomy 21), the occurrence of celiac disease is also observed somewhat more frequently than in people without this chromosomal peculiarity.
Celiac disease has two peaks of manifestation: one in infancy and one in the fourth decade of life. Women are affected more often than men.
A study with evidence of celiac disease based on blood reserves that were created around 1950 and based on blood samples from a comparable population group in 2005 showed that the incidence rate increased fivefold during this period. In addition, the cohort analysis showed that, viewed in the long term, the risk of death of people with undetected celiac disease and therefore not treated with a gluten-free diet was many times higher than that of people without celiac disease.
causes
In affected people, proteins such as gliadin can pass through the epithelial cell layer of the intestinal mucosa. The enzyme tissue transglutaminase (tTG) located in the endomysium modifies the gliadin peptides , which trigger a local immune response and activate intestinal T cells . Environmental factors such as an infection with the Candida albicans fungus , stress or high alcohol consumption can cause increased activity of the tTG and thus promote the development of celiac disease.
Familial accumulation in first-degree relatives and, in particular, identical twins suggests a hereditary factor in the development of celiac disease. First-degree relatives of celiac patients are also affected by 5 to 15 percent; this proportion is 70 percent for identical twins. In fact, more than 99 percent of affected people also have a certain constellation of so-called histocompatibility antigens (HLA), namely HLA DQ2, DQ7 and DQ8. However, a total of 25 percent of all people have this HLA constellation, with about 98 percent of them tolerating the gluten without problems, they develop a tolerance to the harmful components that the remaining two percent apparently do not achieve. Why this is so cannot yet be answered with certainty. The research focuses on further genetic characteristics, but also on infections as possible co-triggering factors.
Celiac disease can be ruled out up to 99 percent if no corresponding genotype can be detected in a symptomatic patient. Genetic diagnostics are only used in exceptional situations such as B. in the context of genetic counseling or if a histological investigation is not possible with typical symptoms and positive antibodies.
One study suggests that reovirus infections trigger the disease at a young age.
Pathophysiology
In the meantime a number of damaging parts of the gluten protein have been precisely identified. They all belong to the alcohol-soluble fraction (so-called prolamins). B. in wheat gliadin , in rye secalin, in barley called hordein and contain a particularly high amount of proline and glutamine as amino acids . In appropriately predisposed people, these protein segments ( peptides , chains of 50–100 amino acids) lead to a complex reaction of the intestinal mucosa and the immune system . Mucous membrane cells of the small intestine ( enterocytes ) increasingly produce different HLA classes (HLA I, DR and DQ). Certain sections of the gluten (gliadin peptides) bind to the increased HLA-DQ2 antigens. This bond is strengthened by the fact that glutamic acid is formed from the numerous amino acid glutamine present in the peptide . This production of glutamic acid is mediated by the enzyme tissue transglutaminase . With this change, the corresponding section of the gliadin fits better into the “pockets” of the HLA proteins. The complex of gliadin peptide and HLA-DQ2 antigen in turn binds to special lymphocytes (CD4 + T helper cells ) and causes an increased production of various inflammatory messenger substances ( interferon-γ , TNF-α , interleukin-6 and interleukin-2 ) .
In the further process of inflammation, various antibodies are formed, of which it is not yet known whether they are causally involved in the development of celiac disease or other autoimmune diseases associated with celiac disease . In addition to antibodies against the gluten itself (gliadin antibodies, AGA), so-called autoantibodies against the body's own antigens also occur. In 1997, tissue transglutaminase was identified as the main autoantigen responsible. Based on these findings, celiac disease is understood from a pathophysiological point of view as a hybrid of allergy and autoimmune disease. The allergic component in the form of hypersensitivity to the exogenous protein gliadin is the triggering factor, while the autoimmune reaction against the body's own structures is responsible for the expression of the symptoms. Ultimately, the inflammatory process ends in a programmed cell death ( apoptosis ) of enterocytes, which eventually became a more or less pronounced loss of thin villi (villi atrophy ) leads. The mucous membrane of the small intestine damaged in this way is no longer able to transfer sufficient amounts of the supplied food into the bloodstream because of the reduced absorption area.
Symptoms
The degree of sensitivity to gluten and the intensity of the symptoms varies from person to person. Some people show only mild symptoms when consuming larger amounts of foods containing gluten. On the other hand, there are sufferers who react to even the slightest trace of gluten with severe symptoms.
The disease is often discovered in early childhood, when the first cereal foods are introduced. This usually shows the classic appearance of celiac disease with noticeable failure to thrive. The classic symptoms of celiac disease are chronic diarrhea caused by the digestive disorder, sometimes with large, foul-smelling stools and, due to the disturbed fat digestion , shiny, sticky stools ( steatorrhea ). Affected children have no appetite, often vomit and do not gain weight or gain enough weight. Later on, the growth in length can also be impaired, the pediatrician speaks of failure to thrive . The children are disgruntled and stand out because of their thin arms and legs and especially their bulging belly.
Often the suspected diagnosis of celiac disease in childhood is made by the dentist if parents observe enamel defects on the teeth during the eruption of permanent teeth. They include tooth discoloration with white, yellow, or brown spots on the teeth, enamel hypoplasia , blotchy or translucent looking teeth. The malformations of the teeth often occur symmetrically on the incisors and molars . They are to be distinguished from other tooth damage that can show similar symptoms. The tooth damage is permanent and does not subside even after starting a gluten-free diet. In addition, recurrent aphthous stomatitis occurs, as well as ulcers , an atrophic glossitis , which is characterized by a red, smooth, shiny tongue and can be associated with burning tongue (burning mouth syndrome). Squamous cell carcinoma of the throat or the lining of the mouth occurs very rarely .
In adults or adolescents, the symptoms are often less pronounced. Here there are silent forms with diffuse complaints. These include B. chronic tiredness and general feeling of illness, weakness, nervousness, sore bones, dry skin or anemia .
Since the examination of celiac disease-specific antibodies in the blood was introduced into diagnostics , the recognition of the clinical picture has fundamentally changed. The S2k guideline on celiac disease 2014 differentiates between classic , symptomatic , subclinical , potential and refractory celiac disease. People who have celiac disease, for example, in the context of family examinations and who have almost complete villus atrophy, can show the typical symptoms ( classic celiac disease ), but can also show only weak and sometimes unspecific symptoms ( symptomatic celiac disease ). The fact that only some of them show the typical symptoms of classic celiac disease has entered the literature under the term “iceberg phenomenon”. They experience abdominal pain, paradoxically even constipation , growth retardation and delayed puberty in children, reduction in the calcium content of the bones ( osteopenia ) due to reduced calcium absorption ( hypocalcemia in the blood serum ), iron deficiency anemia due to reduced iron absorption, joint inflammation , respiratory infections , defects in tooth enamel and psychological abnormalities ( Concentration disorders, depression).
In addition to classic and symptomatic celiac disease, a distinction is made between other forms of disease: In subclinical celiac disease , patients have (almost) complete villus atrophy, but have little or no unspecific symptoms, especially no signs of a nutritional disorder. In potential celiac disease there are immunological deviations typical of celiac disease, but no villus atrophy. (Previously, this term was reserved for people who, despite the immunological deviations typical of celiac disease, never exhibited the classic changes in the mucous membrane of the small intestine, whereas the term latent celiac disease was used when villous atrophy had previously existed with foods containing gluten, but this was able to normalize again with a gluten-free diet and remained normal with a renewed gluten-containing diet.)
| OSLO classification of celiac disease (according to S2k guideline 2014) | |||||
|---|---|---|---|---|---|
| Malabsorption syndrome |
Unspecific symptoms | Celiac specif. AK tTG-AK |
HLA DQ2 DG8 |
Marsh 2 or 3 |
|
| Classic 1 | + | +/- | + | + | + |
| Symptomatic 2 | - | + | + | + | + |
| Subclinical 3 | - | - | + | + | + |
| Potential 4 | - | - | + | + | - |
| Refractory 5th | + | +/- | + | + | + |
|
Previously: 1 typical - 2 atypical / overt - 3 subclinical / asymptomatic / silent - 4 potential / latent 5 only in adults |
|||||
Comorbidities
As an autoimmune disease, celiac disease is often associated with other diseases in which the immune system is directed against the body's own tissue. The most common comorbidities of celiac disease are various neurological complaints which, due to their independent pathogenesis, cannot be seen as symptoms of celiac disease, such as gluten ataxia . The second most common concomitant disease is type 1 diabetes mellitus , in which antibodies are formed against the insulin-producing cells of the pancreas. About five to ten percent of all people with celiac disease also have type 1 diabetes and, conversely, about the same proportion of type 1 diabetics have celiac disease. An inflammatory disease of the thyroid gland, Hashimoto's thyroiditis , which is also considered an autoimmune disease, has also been described as a reciprocal occurrence together with celiac disease. Dermatitis herpetiformis Duhring , a vesicular rash that causes severe itching , is more likely to develop in adults , with only about a tenth of these patients having gastrointestinal symptoms.
Diagnosis
The fact that celiac disease is now considered a widespread disease that affects an estimated one percent of the population in industrialized nations is due, among other things, to the fact that there are now powerful serological tests that can also be used to diagnose atypical forms of the disease. Since it has been shown that a large proportion of celiac disease patients do not suffer from the classic gastrointestinal symptoms, the European Society of Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) has expanded its guidelines for the diagnosis of gluten-induced enteropathies and the specific serological tests recorded. To confirm the suspicion of celiac disease, however, the diagnosis must always be confirmed by an endoscopic tissue biopsy ( see histology ) from the lower duodenum.
The decisive initial suspicion arises from a careful survey of the previous history ( anamnesis ) with inquiries about unspecific complaints and the findings during the physical examination. If a celiac disease is to be further investigated, various antibodies in the blood can first be examined.
Serological diagnostics
In celiac disease there are immunological reactions against a foreign antigen - the gliadin from grain (wheat) - and against autoantigens in the endomysium (this is the loose, collagen- and lattice-fibrous connective tissue between the skeletal muscle fibers, rich in blood capillaries), in primarily against the enzyme tissue transglutaminase (tTg). Celiac disease is therefore a food allergy and an autoimmune disease at the same time and can be detected using gliadin antibodies and autoantibodies against endomysium or tTg.
One difficulty in serological detection of celiac disease is that around ten percent of the affected patients are IgA deficient. This means that no IgA antibodies can then be detected. However, this problem can be avoided by not only determining the IgA antibodies, but also by carrying out the somewhat less specific IgG detection.
Anti-endomysium
Autoantibodies against endomysial antigens are highly specific and can be detected in over 90 percent of patients with celiac disease. This is done using an indirect immunofluorescence test on tissue sections from monkey esophagus ( esophagus = esophagus). This technique requires specially trained laboratory personnel who are experienced in evaluating the immunofluorescence patterns.
The anti-endomysial concentrations reflect the histological appearance: the higher the antibody titers , the more pronounced the villous atrophy. In addition, the anti-endomysial titers also decrease significantly if a gluten-free diet is followed. Like the gliadin antibodies ( see Gliadin antibodies ), they can also be helpful in monitoring therapy.
Anti-tissue transglutaminase
The anti-endomysium detection by means of immunofluorescence technology requires special technical skills on the part of the user, takes a long time and requires relatively rare biological material. That is why it was a big step forward when the enzyme tissue transglutaminase (tTg, German: tissue transglutaminase) was identified as the main antigen in the endomysium.
tTg belongs to a family of calcium-binding enzymes that connect glutamine and lysine residues in polypeptide chains and thus cross-link proteins. The detection of tTG antibodies in ELISA has meanwhile developed into the gold standard in celiac disease diagnostics.
Current clinical studies show that an anti-tissue transglutaminase screening in the ELISA, which simultaneously detects IgA and IgG antibodies, is a sensitive and specific alternative to the detection of endomysial antibodies with immunofluorescence, and favor a tTG ELISA as diagnostic tool for celiac disease.
Gliadin antibodies
Detecting antibodies to gliadin was historically the first way to detect celiac disease using an antibody test. The detection of gliadin antibodies is very sensitive, especially if both IgG and IgA antibodies are determined, but the detection is not very specific. Gliadin antibodies are also found in allergy sufferers (atopics) or people with other autoimmune diseases and in around five percent of the healthy population.
Gliadin antibodies therefore only play a subordinate role in the diagnosis of celiac disease. However, they are still important for therapy monitoring: if a gluten-free diet is strictly adhered to, the anti-gliadin concentrations decrease at the same time as the symptoms of the disease subside.
Since 2004, numerous serological tests have been tried in which various forms of gliadin are used as target antigens. It remains to be seen whether these tests, which are usually produced using genetic engineering, are as powerful and as specific as the established detection of antibodies against tTG. According to the guidelines of ESPGHAN, the result of the serological tests must always be confirmed by a small intestine biopsy in order to be able to make the final diagnosis.
Usually antibodies against gliadin of the IgA and IgG type (AGA IgA and IgG), endomysial antibodies (EMA) of the IgA type (= autoantibodies against tissue transglutaminase (tTG-A)) are determined. The latter have the highest specificity with 87.4–98.2% (if the test result is positive, there is actually a disease) and with 86.5 to 97.2% the highest sensitivity (a high proportion of the sick is recognized by the test) on. However, the tTG antibodies are always of the IgA type. However, since up to 11 percent of celiac patients are unable to produce sufficient IgA at the same time (IgA deficiency), the total IgA concentration must always be determined so that false negative results are not overlooked. It must also be borne in mind that the sensitivity of the EMA is only around 80 percent in children under two years of age. At this age, therefore, the gliadin antibodies (AGA of the IgA and IgG type) are of particular diagnostic importance. The antibody determinations are also suitable for follow-up monitoring on a gluten-free diet, as their concentrations fall below the detection limit with increasing duration of therapy.
histology
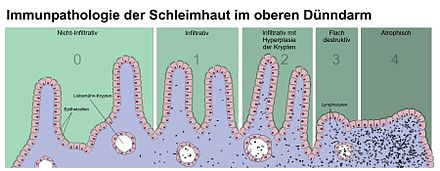
If the suspicion of celiac disease is corroborated by positive antibody findings, the diagnosis must be confirmed by a subsequent small intestine biopsy in accordance with the recommendations of the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) . In this process, one, preferably several, small samples of the mucous membrane are taken from the descending portion of the duodenum - nowadays mostly by means of a gastric and partial small intestine specimen ( gastroduodenoscopy ) . It is not always possible to find the appropriate location for the biopsy. The removed tissue pieces by a pathologist fine tissue examined at the microscope, the structure of the small intestinal mucosa is judged at first at low magnification (50X). With a higher magnification, it is then examined whether the mucous membrane shows an increase in inflammatory cells. For the diagnosis of celiac disease, the lymphocytes located within the covering layer ( epithelium ) are decisive. In the case of borderline findings, the histological standard staining can be expanded to include an immunohistology , with which the intraepithelial lymphocytes can be better quantified. The gold standard for assessing the small intestine biopsy are the so-called Marsh criteria: number of lymphocytes that have migrated into the mucous membrane, villi length in relation to the crypts, cell division rate, number of inflammatory cells in the lamina propria (intestinal wall layer directly below the epithelium) and assessment of the brush border in one special staining ( PAS staining ). The Marsh 2 or Marsh 3 criteria are required for a diagnosis of celiac disease:
- Marsh 2: ≥ 40 intraepithelial lymphocytes / 100 enterocytes + crypt hyperplasia
- Marsh 3: ≥ 40 intraepithelial lymphocytes / 100 enterocytes + crypt hyperplasia + villus atrophy.
Another, for example infectious, cause of the inflammation should be ruled out by searching for microorganisms such as Tropheryma whipplei , Giardia and Cryptosporidia . The diagnostic criteria of ESPGHAN, revised in 1990, also demand a clear clinical improvement after initiation of therapy. Control biopsies, which used to be common, are therefore only indicated for special indications such as doubtful clinical success with a gluten-free diet, doubts about the initial diagnosis or to differentiate between temporary gluten intolerance.
Differential diagnosis
Although the clinical picture is quite clear with a typical course, even an apparently typical, but not specific histology of the small intestinal mucosa still results in some possible differential diagnoses . Food allergies (especially cow's milk and soy protein) or various infections of the intestinal tract can also lead to similar damage to the mucous membranes. Other rare causes of food intolerance, diarrhea, etc. a. are other autoimmune gastrointestinal diseases, immunodeficiencies , AIDS , rejection reactions after transplantation, radiation or treatment with cytostatic drugs , significant malnutrition or the very rare microvillus atrophy in small children.
Another important differential diagnosis is cystic fibrosis , which should be excluded by the sweat test (pilocarpine iontophoresis test) based on the chloride content measured in sweat. Furthermore, congenital pancreatic insufficiency , congenital intestinal enzyme defects (e.g. lactase or sucrose deficiency), tropical sprue , collagenous sprue, Crohn's disease and Whipple 's disease should be considered.
If in doubt, a gluten-free diet can be suggested on a trial basis. Success and the symptoms disappear would be an indication of celiac disease or a more common intolerance to other proteins.
Risk patients
In the case of the following problems, special attention should be paid to whether celiac disease may be present: 1st degree relatives of celiac patients, type 1 diabetes mellitus , selective IgA deficiency , Down's disease (trisomy 21), Turner syndrome , Williams-Beuren syndrome , Duhring herpetiform dermatitis , Vitiligo , autoimmune thyroid disease , autoimmune hepatitis , rheumatoid arthritis .
Prevention
In order to minimize the risk of developing celiac disease, the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) recommends, based on study data, not to use gluten in infant nutrition before they are four months old.
A study from 2019 shows that a high-fiber diet for the expectant mother during pregnancy can lower the child's risk of celiac disease.
treatment
The only sure way to treat the disease is a lifelong gluten-free diet , which allows the intestinal mucosa to recover and the risks of long-term consequences are reduced. At the same time, care should be taken to eat a nutrient-rich diet, as the damage to the small intestine can easily lead to deficiencies in important nutrients. Strictly avoid all types of grain with a high gluten content ( wheat , barley , rye as well as their botanically related original varieties spelled , green spelled , kamut , einkorn , emmer and the rye-wheat cross triticale ). So far, the avoidance of the grass genus oats has also been recommended, although the chemical composition of the prolamins differs from that of wheat and oats in Finland and England have been released in moderate quantities and under medical supervision for adult people with celiac disease. Particularly with processed foods and finished products, care must be taken to ensure that no gluten-containing ingredients have been used. Since gluten is often used as an emulsifier , for gelling, stabilizing and as a carrier of flavorings, this is not always easy to recognize. An overview of gluten-free foods is available from Deutsche Zöliakiegesellschaft e. V. (DZG).
Millet , corn , rice , water rice (“wild rice”), amaranth , tapioca , buckwheat , quinoa , soybeans , teff , chestnut and plantain are suitable alternatives to cereals containing gluten . Some of these types are also used to make gluten-free beer , for example . Are already allowed vegetables and potatoes , salads , fruits , meat and fish , eggs , milk and dairy products .
Researchers have been able to develop a genetically modified wheat whose flour can still be baked in bread without the gluten proteins that are relevant for celiac disease. The relevant gene sequences for protein expression of gluten have been removed from the genome of the Chinese Spring wheat variety , so that the epitopes of the gluten proteins can no longer be recognized by the human antibodies.
Gluten-free special foods were initially only available in health food stores , later also in specialty stores for gluten-free nutrition and in individual regular grocery stores. Gluten-free fresh and finished products can now also be found in general grocery stores.
According to the Codex Alimentarius Standard 118-1981, the limit value for gluten-free products is 20 ppm (= 20 mg / kg). Gluten-free foods produced in Germany generally comply with the 20 ppm limit. As the lactose digestion can also be impaired in some patients at the beginning of therapy due to extensive damage to the mucous membrane (secondary lactose intolerance ), they must temporarily ensure a diet low in lactose . In particular, milk and dairy products can be replaced by soy milk .
research
A research approach presented in 2006 to develop another treatment option for celiac disease consisted of enzyme replacement therapy . Enzymes isolated from germinating grain or a fungus could cut the gluten into small pieces so that the fragments could no longer be recognized by the immune system and, accordingly, no more inflammation could be triggered. (There were no further publications on this therapy until 2014, and the AWMF guideline from 2009 does not mention this form of therapy either.)
A combination of a glutamine-specific endoprotease (EP-B2 from barley) and a prolylendopeptidase (SC PEP from Sphingomonas capsulata) is mentioned as an enzyme combination that could enable gluten to be digested under intestinal conditions.
A laboratory method presented in 2011 allows the evaluation of various enzymes ( prolyl endopeptidase ) with regard to stability and effectiveness in the digestive tract to be followed in real time. Thanks to this procedure, some crucial differences between enzymes of a similar nature have been identified, which have remained hidden during investigations in the test tube.
Some of the treatment approaches are the subject of Phase II clinical trials .
EU regulation
Regulation (EC) number 41/2009 dealt with the composition and labeling of foods (exception: infant formula and follow-on formula) that are suitable for people with gluten intolerance. The EU regulation permitted certain maximum levels of gluten in the food in question, as it is technically very difficult to produce completely gluten-free food. According to this regulation, possible declaration levels for foods that are offered for people with gluten intolerance were:
- “ Very low gluten content ”: a maximum of 100 mg of gluten per kilogram of food may be present
- " Gluten -free ": The maximum gluten content is 20 mg / kg
- " Food with oats ": a maximum of 20 mg / kg gluten. The oats must be produced in such a way that contamination with barley, rye, wheat and their hybrids is excluded.
This regulation came into force on January 1, 2012, but was repealed by Regulation (EU) No. 609/2013 of June 12, 2013.
history
The term celiac disease is derived from the ancient Greek κοιλία koilia , German 'belly' , 'lower body' (from κοίλος koilos , German 'hollow' ). The "bulbous disease" was mentioned by Aretaios of Cappadocia as early as the second century AD. Christian Henrich Heineken died in 1725 after several months of suffering, presumably of celiac disease, which was unknown at the time. In general, Samuel Gee is considered to be the first person to describe the disease. In 1888 he reported about the "celiac affection" and meant a digestive disorder that mainly affected small children. It was not until 1950 that Willem Karel Dicke finally identified wheat gliadin as a decisive harmful factor. Dicke published his first studies as early as 1941 after he noticed sudden improvements in the condition of children suffering from celiac disease (known at the time as Gee-Herter syndrome) after they hardly received any cereal products as a result of the inadequate supply during the war. Villi atrophy was first described by Margot Shiner from London in 1957. A year later, with the first description of the gliadin antibodies by E. Berger from Basel, serological diagnostics were introduced.
The new findings about the clinical picture led to the adoption of the diagnostic criteria for celiac disease by the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN), the so-called "Interlaken criteria" for the first time in 1969. These apply today in the revised version from 1990. The discovery of the endomysial antibodies as a highly specific serological marker dates back to the 1980s. Finally, in 1997, tissue transglutaminase (tTG) was recognized as the key antigen for these antibodies.
Others
In 2016 the project "Focus IN CD" - Innovative patient centered health care services - advantages of establishing a close CE network in celiac disease patient health care "started, which was mainly funded by the European regional development program" Interreg Central Europe "and other non-profit organizations is funded over a period of 36 months (project number CE111). A total of 12 partner institutions from the fields of medicine and research, patient self-help, project development and administration are involved, who implement the project in Slovenia, Germany, Hungary, Croatia and Italy. Project partners in Germany are the Clinic of the University of Munich ( Dr. von Haunersches Kinderspital ) and the Children's Health Foundation . The main goal of Focus IN CD is to use various sub-projects to significantly and sustainably improve the care of patients with celiac disease in Central Europe. In particular, the transfer of knowledge about celiac disease and The focus is on a gluten-free diet in order to better inform doctors and medical staff as well as the patients themselves about celiac disease and thus create more safety for those affected by celiac disease. For this purpose, a first online course for patients with celiac disease was published in 2018 with the title Zoeliakie-verhaben.de, which clearly presents detailed, independent and current information on celiac disease.
Since 2002, World Celiac Day (WZT) has usually taken place on the third weekend in May . It was launched by the umbrella organization of the European Celiac Societies ( AOECS - Association of European Celiac Societies). On this day, the topic of "Celiac disease and gluten-free nutrition" is to be brought closer to the broadest possible public with a variety of activities. In Germany, the WZT is organized by the German Celiac Society (DZG).
In addition, the gluten-free Oktoberfest takes place every second year, alternating with World Celiac Day, which is also organized by the German Celiac Society (DZG).
See also
- Gluten ataxia , a neurodegenerative autoimmune disease characterized by movement disorders
- Dermatitis herpetiformis Duhring , blistering skin disease with itching
- Non-Celiac Disease-Non-Wheat Allergy-Wheat Sensitivity , still largely a diagnosis of exclusion
literature
- German Society for Gastroenterology, Digestive and Metabolic Diseases (DGVS): Celiac disease, wheat allergy and wheat sensitivity ; As of April 30, 2014, valid until April 30, 2019
- W. Holtmeier, WF Caspary: Celiac disease. In: Orphanet Journal of Rare Diseases. London 1.2006, 3rd doi: 10.1186 / 1750-1172-1-3 PMID 16722573 ISSN 1750-1172 ( Open Access ).
- Alessio Fasano: Autoimmune Diseases. Fatal bowel disease celiac disease . In: Spectrum of Sciences May 2010 (pdf, accessed June 11, 2018).
- W. Kiess: Therapy in child and youth medicine. Urban & Fischer, Elsevier, Munich 2007, ISBN 978-3-437-23200-8 .
Web links
- Microscopic preparation at Sprue on PathoPic
- Scientific review article by the American Academy of Family Physicians (English)
- Website of the German Celiac Society
- Website of the Austrian Working Group on Celiac Disease
- Website of the Society for Pediatric Gastroenterology and Nutrition
Individual evidence
- ↑ www.netdoktor.de: Zoeliakie .
- ↑ JF Ludvigsson, F. Zingone, T. Tomson, A. Ekbom, C. Ciacci: Risk of epilepsy in celiac disease . In: Neurology . tape 78 , no. 18 , May 1, 2012, p. 1401-1407 , doi : 10.1212 / WNL.0b013e3182544728 (English).
- ↑ German Society for Gastroenterology, Digestive and Metabolic Diseases (DGVS): Celiac disease, wheat allergy and wheat sensitivity ; Guideline; As of April 30, 2014, valid until April 30, 2019, p. 42.
- ↑ Celiac disease , duden.de
- ↑ celiacus , zeno.org
- ↑ Celiaki och gluten , kotus.fi (in Swedish)
- ↑ Jacqueline Coutts, Richard Fielder: Management of Food Allergens . Wiley-Blackwell, ISBN 1-4051-6758-0 , pp. 157 ff .
- ↑ https://www.gen-ethisches-netzwerk.de/zukunft-kleine-brotchen
- ↑ a b c d e f R. Keller: Clinical symptoms “Celiac disease, an iceberg”. In: Monthly Pediatrics. Heidelberg 151.2003, 706-714. ISSN 0026-9298
- ↑ a b c K. P. Zimmer: Pathophysiology of Celiac Disease. In: Monthly Pediatrics. Heidelberg 151.2003, 698-705. ISSN 0026-9298
- ↑ Alberto Rubio – Tapia, Robert A. Kyle, Edward L. Kaplan, Dwight R. Johnson, William Page, Frederick Erdtmann, Tricia L. Brantner, W. Ray Kim, Tara K. Phelps, Brian D. Lahr, Alan R. Zinsmeister, L. Joseph Melton, Joseph A. Murray: Increased Prevalence and Mortality in Undiagnosed Celiac Disease. In: Gastroenterology. Vol. 137, No. 1, 2009, pp. 88-93, ISSN 0016-5085 , doi: 10.1053 / j.gastro.2009.03.059
- ↑ MF Kagnoff: Celiac disease: pathogenesis of a model immunogenetic disease. In: J Clin Invest. 2007; 117 (1), pp. 41-49.
- ↑ Jürgen F. Riemann : Gastroenterology: the reference work for clinics and practices. Georg Thieme Verlag, 2007, ISBN 978-3-13-141201-0 , p. 681.
- ↑ a b Diagnosis and therapy of celiac disease. In: Deutsches Ärzteblatt Online. 2013, accessed February 3, 2014.
- ↑ Romain Bouziat, Reinhard Hinterleitner, Judy J. Brown, Jennifer E. Stencel-Baerenwald, Mine Ikizler: Reovirus infection triggers inflammatory responses to dietary antigens and development of celiac disease . In: Science . tape 356 , no. 6333 , April 7, 2017, ISSN 0036-8075 , p. 44–50 , doi : 10.1126 / science.aah5298 , PMID 28386004 ( sciencemag.org [accessed April 11, 2017]).
- ^ Dental Enamel Defects and Celiac Disease ( Memento of March 5, 2016 in the Internet Archive ), The National Institutes of Health Celiac Disease Awareness Campaign. NIH Publication No. 11-7397, April 2011. Retrieved June 17, 2016.
- ↑ a b S2k Celiac Disease Guideline: New Guideline 2014. DGVS, accessed on December 14, 2014 . P. 15.
- ^ Marsh classification of celiac disease. DZG, accessed on December 14, 2014 .
- ↑ Elizabeth Mearns, Aliki Taylor, Kelly Thomas Craig, Stefanie Puglielli, Daniel Leffler: Neurological Manifestations of Neuropathy and Ataxia in Celiac Disease: A Systematic Review . In: Nutrients . tape 11 , no. 2 , February 12, 2019, ISSN 2072-6643 , p. 380 , doi : 10.3390 / nu11020380 , PMID 30759885 , PMC 6412791 (free full text) - ( mdpi.com [accessed July 20, 2020]).
- ^ Giovanni Casella, Bianca M. Bordo, Renzo Schalling, Vincenzo Villanacci, Marianna Salemme: Neurological disorders and celiac disease . In: Minerva Gastroenterologica E Dietologica . tape 62 , no. 2 , June 2016, ISSN 1827-1642 , p. 197-206 , PMID 26619901 ( nih.gov [accessed July 20, 2020]).
- ↑ Green PH, Cellier C.: Celiac disease. In: N Engl J Med. 2007; 357 (17), pp. 1731-1743.
- ↑ espghan.med.up.pt ( Memento of the original from September 9, 2010 in the Internet Archive ) Info: The archive link has been inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. European Society of Pediatric Gastroenterology and Nutrition website
- ↑ D. Villalta, MG Alessio, M. Tampoia et al: Testing for IgG class antibodies in celiac disease patients with selective IgA deficiency. A comparison of the diagnostic accuracy of 9 IgG anti-tissue transglutaminase, 1 IgG anti-gliadin and 1 IgG anti-deaminated gliadin peptide antibody assays . In: Clin. Chim. Acta . tape 382 , no. 1-2 , January 2007, pp. 95-99 , doi : 10.1016 / j.cca.2007.03.028 , PMID 17490629 .
- ↑ Schwertz et al: Serological assay based on gliadin-related nonapeptides as a highly sensitive and specific diagnostic aid in celiac disease. In: Clin Chem. 2004; 50, pp. 2370-2375.
- ↑ D. Agardh: Antibodies against synthetic deamidated gliadin peptides and tissue transglutaminase for the identification of childhood celiac disease. In: Clin Gastroenterol Hepatol . 2007; 5 (11), pp. 1276-1281.
- ↑ S. Buderus, MJ Lentze: Serological diagnosis of celiac disease. In: Monthly Pediatrics. Heidelberg 151.2003, pp. 715-718. ISSN 0026-9298
- ↑ Acids - Bases - Slags . Springer, Vienna 2007, ISBN 978-3-211-29133-7 .
- ↑ Monika Kovacsics: Causes of gluten intolerance. Odysso, September 29, 2011; 3sat Nano, February 8, 2012.
- ↑ awmf.org ( Memento from May 24, 2012 in the Internet Archive )
- ↑ Hania Szajewska et al .: Gluten Introduction and the Risk of Celiac Disease: A Position Paper by the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. In: Journal of Pediatric Gastroenterology and Nutrition 2016 Mar; 62 (3): 507-513. doi: 10.1097 / MPG.0000000000001105 , PMID 26815017 , http://www.espghan.org/fileadmin/user_upload/guidelines_pdf/Hep_Nutr/Gluten_Introduction_and_the_Risk_of_Coeliac.32.pdf .
- ↑ James Butcher: High fiber during pregnancy reduces risk of celiac disease in children, research finds. In: EurekAlert! June 6, 2019, accessed June 16, 2019 .
- ↑ a b A. van Teeffelen-Heithoff: Dietary basics of celiac disease treatment. In: Monthly Pediatrics. Heidelberg 151.2003, pp. 719-725. ISSN 0026-9298 , doi: 10.1007 / s00112-003-0750-x
- ↑ Overview of the selection of gluten-free foods (PDF). Retrieved August 28, 2019
- ↑ DZG currently . 2005.1, p. 29. ISSN 0947-5222
- ↑ Hetty C van den Broeck, Teun WJM van Herpen, Cees Schuit, Elma MJ Salentijn, Liesbeth Dekking, Dirk Bosch, Rob J Hamer, Marinus JM Smulders, Ludovicus JWJ Gilissen, Ingrid M van der Meer: Removing celiac disease-related gluten proteins from bread wheat while retaining technological properties: a study with Chinese Spring deletion lines. In: BMC Plant Biology. 9, 2009, p. 41, doi: 10.1186 / 1471-2229-9-41
- ↑ CODEX STANDARD FOR FOODS FOR SPECIAL DIETARY USE FOR PERSONS INTOLERANT TO GLUTEN. (PDF; 39 kB) CODEX STAN 118-1979. In: Codex Alimentarius. Archived from the original on September 15, 2012 ; accessed on November 16, 2012 (English).
- ↑ Süddeutsche Zeitung . Munich 176.2006, p. 16. ISSN 0174-4917
- ↑ Celiac Disease. ( Memento of May 24, 2012 in the Internet Archive ) Guidelines of the Society for Pediatric Gastroenterology and Nutrition 2009, accessed on February 3, 2014.
- ↑ Jonathan Gass, Michael T. Bethune, Matthew Siegel, Andrew Spencer, Chaitan Khosla : Combination Enzyme Therapy for Gastric Digestion of Dietary Gluten in Patients With Celiac Sprue. In: Gastroenterology. Vol. 133, No. 2, 2007, pp. 472-480, ISSN 0016-5085 , PMID 17681168 , doi: 10.1053 / j.gastro.2007.05.028
- ↑ G. Fuhrmann, JC Leroux: In vivo fluorescence imaging of exogenous enzyme activity in the gastrointestinal tract. In: Proceedings of the National Academy of Sciences. 108 (2011), pp. 9032-9037 (online)
- ^ Rohini R. Vanga: Novel Therapeutic Approaches for Celiac Disease. In: Discovery Medicine. May 22, 2014.
- ↑ Marja-Leena Lähdeaho, Katri Kaukinen, Kaija Laurila, Pekka Vuotikka, Olli-Pekka Koivurova, Tiina Kärjä-Lahdensuu, Annette Marcantonio, Daniel C. Adelmanm Markku Mäki: Glutenase ALV003 Attenuates Gluten-Induced Disease With Patients. In: Gastroenterology. Vol. 146, No. 7, 2014, pp. 1649-1658, ISSN 0016-5085 , PMID 24583059 , doi: 10.1053 / j.gastro.2014.02.031
- ↑ Regulation (EC) No. 41/2009
- ↑ Regulation (EU) No. 609/2013
- ↑ Willem Karel Dicke: Een onderzoek naar de nadelige invloed van sommige graansoorten op de lijder aan Coeliakie. Dissertation . Utrecht 1950.
- ^ Willem Karel Dicke: Simple dietary treatment for the syndrome of Gee-Herter. In: Nederlands Tijdschrift voor Geneeskunde. Volume 85 (1941), pp. 1715-1716.
- ↑ Focus IN CD: Focus IN CD - a project to improve the management of celiac disease care in central Europe. (No longer available online.) Focus IN CD, formerly the original ; accessed on January 22, 2019 (English). ( Page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice.
- ↑ Focus IN CD: Understanding Celiac Disease. Retrieved January 22, 2019 .
- ^ DZG: World Celiac Day - DZG campaigns in Germany. Retrieved January 22, 2019 .
- ↑ Gluten-free Oktoberfest. Retrieved January 22, 2019 .