menthol
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| Structural formula without stereochemistry | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | menthol | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 10 H 20 O | |||||||||||||||||||||
| Brief description |
colorless, shiny prisms |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 156.27 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
0.89 g cm −3 |
|||||||||||||||||||||
| Melting point |
31, 33, 35 and 42.5–43 ° C [(-) - menthol, 4 modifications] |
|||||||||||||||||||||
| boiling point |
212 ° C |
|||||||||||||||||||||
| solubility |
Slightly soluble in water, readily soluble in ethanol , diethyl ether and chloroform |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Menthol is a monocyclic monoterpene - alcohol . It exists in two mirror-image forms, (-) - menthol ( levomenthol ) and (+) - menthol. In addition to menthol, there are three other pairs of diastereomers (also called "isomeric mentholes"), as the compound has three asymmetric carbon atoms: neomenthol , isomenthol and neoisomenthol .
The natural, levorotatory (-) - menthol is found in many essential oils , especially mint oils .
Occurrence

(-) - Menthol occurs naturally in the essential oil of plants of the genus Mentha ; in "Japanese peppermint oil" from grown in China or Japan corn mint ( Mentha arvensis ) are up to 90% by weight. In other genera and species of the family Lamiaceae ( Labiatae ) there are menthol, so the spice plant basil ( Ocimum basilicum ), marjoram ( Origanum majorana ), oregano ( Origanum vulgare ), rosemary ( Rosmarinus officinalis ), sage ( Salvia ) and thyme ( thymus ). (+) - Neomenthol is found in Japanese peppermint oil, (-) - Neoisomenthol with up to one percent in geranium oil .
Extraction and presentation
Over 19,000 tons of (-) - menthol are produced worldwide every year, around two thirds of which are obtained from plants and one third is produced synthetically. Vegetable production takes place by freezing out the crystalline menthol from the essential oil of the field mint . The technical synthesis of (-) - menthol is currently carried out using various processes at the companies Symrise (previously Haarmann & Reimer , from 1973), Takasago (from 1984) and BASF (from 2012).
The picture shows a technical synthesis of (-) - menthol. It is based on citronellal ( 1 ) to which zinc bromide is added. In a carbonyl-ene reaction, it is converted to isopulegol ( 2 ). This is hydrogenated to menthol ( 3 ) on nickel catalysts . But this is only one of many menthol syntheses: Menthol can also be synthesized from pulegone , phellandrene , 3-carene , pinene , limonene , myrcene , piperitone or by hydrogenation from thymol or cresol .
Properties and isomerism
At room temperature, menthol is a colorless, crystalline solid with a peppermint odor . The symmetry of the crystal lattice is trigonal, the crystals are needle-shaped.
2-Isopropyl-5-methylcyclohexanol has three stereogenic centers, therefore there are eight stereoisomers :
| Isomers of menthol | ||||||||
|---|---|---|---|---|---|---|---|---|
| Surname | (+) - menthol | (-) - menthol | (+) - isomenthol | (-) - isomenthol | (+) - neomenthol | (-) - Neomenthol | (+) - neoisomenthol | (-) - Neoisomenthol |
| other names | D -Menthol | Levomenthol,
L -Menthol |
D -isomenthol | L-isomenthol | D -neomenthol | L -neomenthol | D -neoisomenthol | L -neoisomenthol |
| (±) -menthol, DL -menthol, racementhol
[ DL - (±) mixture] |
DL -isomenthol [ DL - (±) -mixture] | DL -isomenthol [ DL - (±) -mixture] | DL -neoisomenthol [ DL - (±) -mixture] | |||||
| Structural formula |

|
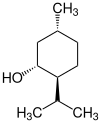
|
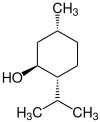
|

|

|

|
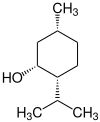
|

|
| Stereocenters | 1 S , 2 R , 5 S. | 1 row , 2 row , 5 row | 1 S , 2 R , 5 R | 1 R , 2 S , 5 S | 1 S , 2 S , 5 R | 1 R , 2 R , 5 S. | 1 row , 2 row , 5 row | 1 S , 2 S , 5 S |
| Melting point | 31, 33, 35 and 42.5-43 ° C
(4 modifications) |
82.5 ° C | ? | -8 ° C | ||||
| ? | 53.5–54.5 ° C [ DL - (±) mixture] | 50–51 ° C [ DL - (±) mixture] | 14 ° C [ DL - (±) mixture] | |||||
| boiling point | 212 ° C | 96.2-96.8 ° C (10 mmHg ) | 102 ° C (19 mmHg) | 105 ° C (12 mmHg) | ||||
| ? | 218.5–218.6 ° C [ DL - (±) mixture] | 212.1–212.6 ° C [ DL - (±) mixture] | 81 ° C (6 mmHg) [ DL - (±) mixture] | |||||
| Rotation value | 49.6 ° (ethanol) | 25.9 ° (ethanol) | 20.7 ° (ethanol) | 2.2 ° (ethanol) | ||||
| CAS number | 15356-60-2 | 2216-51-5 | 23283-97-8 | 20752-33-4 | 2216-52-6 | 20747-49-3 | 20752-34-5 | 64282-88-8 |
| 89-78-1 [ DL - (±) mixture] | 3623-52-7 [ DL - (±) mixture] | 3623-51-6 [ DL - (±) mixture] | 491-02-1 [ DL - (±) mixture] | |||||
All are secondary , monohydric alcohols : the molecule has a hydroxyl group ; only two further carbon atoms are directly bound to the carbon atom to which this hydroxyl group is bound. The international non-proprietary name (INN) of the substance consisting of (-) - menthol and (+) - menthol in an enantiomeric ratio of 1: 1 is racementhol .
The taste threshold is between 0.2 ppm for (-) - menthol and 1 ppm for (+) - menthol.
The stereoisomers differ in terms of their odor , among other things :
- (+) - and (-) - menthol smell fresh, minty and sweet and make up the typical peppermint smell, whereby the smell of (-) - menthol is about three times more pronounced.
- The enantiomers of isomenthol and neomenthol smell rather dull and unpleasant.
- (-) - Neoisomenthol smells like camphor , stale, sweet, minty; (+) - Neoisomenthol has a camphor, stale and forest smell, but it does not smell minty, cool and fresh.
Isomenthol, neomenthol and neoisomenthol also have no cooling effect - which is subjectively felt in the mouth when inhaled.
Responsiveness
The oxidation of menthol [in the picture (-) - menthol] with chromic acid yields menthone [in the picture (-) - menthol ]:
Through the action of concentrated sulfuric acid , menthol [in the picture (-) - menthol] is dehydrated to menthene:
use
Menthol is added to a wide variety of products as a disinfectant and as a fragrance and flavoring agent, such as confectionery (0.05-0.1%) and liqueur (0.1-0.2%), perfume ( 0.05-0.1%) , 4%), body care , dental and oral care products (0.5–2.0%), lotions (0.2–0.3%) and hair lotions (0.2–0.5%).
From a medical point of view, menthol is a component of ointments (up to 6%) and rubs against skin irritations in the case of minor burns, insect bites or itching. It works on the cold menthol receptor ( TRPM8 ). Therefore, menthol creates a cool feeling when applied to the skin , but without actually affecting the body temperature. When nasal cold receptors are irritated, the feeling of easier breathing arises.
Menthol blocks the voltage-dependent sodium channels Na v 1.8 and NA v 1.9. This means that menthol has a local anesthetic effect .
The activation of the GABA A receptor explains the central damping effect.
Menthol also acts as a weak agonist on the κ - opioid receptor (KOR).
Menthol is used in bee care as a remedy against mite infestation .
Tobacco products
Menthol has been added to tobacco products since the 1920s. When inhaling the smoke, it reduces the sensation of irritation and pain in the respiratory tract. It changes the density of nicotine receptors in the central nervous system. In addition, it changes the metabolism of nicotine and increases its bioavailability. It increases nicotine addiction. The placing on the market of tobacco products with added menthol has been prohibited throughout the EU since May 20, 2020; Menthol-flavored pods are still allowed.
Hazard warnings
Menthol is lovely; just a few grams of menthol cause cardiac arrhythmias . There is an additional risk for infants and small children, as they can develop severe dyspnoea with respiratory arrest due to the inhalation of menthol. The mean lethal oral dose (LD 50 ) of (-) - menthol for a rat is 3300 mg / kg.
Metabolism
The metabolism of menthol takes place mainly in the liver through the formation of menthol glucuronide .
literature
- Friedrich Hartmut Dost: Menthol and menthol-containing external remedies . Thieme-Verlag, Stuttgart 1967, DNB 457573562 .
- Anja Langeneckert: Studies on the pharmacokinetics and relative bioavailability of α-pinene, 1,8-cineole and menthol after dermal, inhalative and oral application of essential oils . Shaker Verlag , 1999, ISBN 3-8265-6457-X .
Web links
- Fragrance lexicon at www.chemischemlexikon.de: Menthol / Levomenthol , accessed on June 9, 2013
- Leffingwell & Associates: Menthol - A Cool Place , accessed June 9, 2013
Individual evidence
- ↑ a b c d e f g h Entry on menthol. In: Römpp Online . Georg Thieme Verlag, accessed on August 26, 2013.
- ↑ a b c d Entry on menthol in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ Bernd Schäfer: Natural substances in the chemical industry. Spektrum Akademischer Verlag, 2007, ISBN 978-3-8274-1614-8 , pp. 96-104.
- ^ Wissenschaft-Online-Lexika: Entry on menthol in the Lexicon of Nutrition, accessed on August 26, 2013.
- ↑ Menthol Background & Menthol Enantiomers - Organoleptic Properties. Leffingwell & Associates, accessed August 26, 2013.
- ^ B. Schäfer, Chemistry in Our Time, 2013, 47, 174; doi : 10.1002 / ciuz.201300599 .
- ↑ R. Bombicz, J. Bushmann, P. Luger, Nguyen Xuan Dung, Chu Ba Nam: Crystal structure of (1R, 2S, 5R) -2-isopropyl-5-methyl-cyclohexanol, (-) - menthol. In: Z. Kristallogr. 214, No. 7, 1999, pp. 420-423.
- ^ A b c d John Read, William J. Grubb, David Malcolm: 51. Researches in the menthone series. Part XI. Diagnosis and characterization of the stereoisomeric menthols . In: Journal of the Chemical Society (Resumed) . 1933, ISSN 0368-1769 , p. 170 , doi : 10.1039 / jr9330000170 .
- ^ A b c d e f g John Read, William J. Grubb: 76. Researches in the menthone series. Part XII. Isolation and characterization of the neoisomenthols . In: Journal of the Chemical Society (Resumed) . 1934, ISSN 0368-1769 , p. 313 , doi : 10.1039 / jr9340000313 .
- ^ A b John Read, William J. Grubb: 50. Researches in the menthone series. Part X. The complete optical resolution of dl-neomenthol by means of 1-menthol . In: Journal of the Chemical Society (Resumed) . 1933, ISSN 0368-1769 , p. 167 , doi : 10.1039 / jr9330000167 .
- ↑ Bruno Puetzer, William J. Moran: Separation of l-menthol from racemic menthol USP * . In: Journal of the American Pharmaceutical Association (Scientific ed.) . tape 35 , no. 4 , April 1946, p. 127-128 , doi : 10.1002 / jps.3030350407 .
- ↑ a b Waichiro Tagaki, Takeshi Hashizume: Notes- Synthesis and Properties of Isomeric Menthyl Phosphates. Organophosphorus Compounds III . In: The Journal of Organic Chemistry . tape 26 , no. 8 , August 1961, ISSN 0022-3263 , p. 3038-3040 , doi : 10.1021 / jo01066a644 .
- ↑ a b c d K. Hardtke et al. (Ed.): Commentary on the European Pharmacopoeia Ph. Eur. 4.0, Menthol . Loose-leaf collection, 20th delivery 2005, Wissenschaftliche Verlagsgesellschaft Stuttgart.
- ^ H. Wagner: Pharmaceutical Biology. 2nd Edition. WVG, 1981, p. 48.
- ↑ HC Brown, CP Garg: A simple procedure for the chromic acid oxidation of alcohols to ketones of high purity. In: J. Am. Chem. Soc. 83, 1961, pp. 2952-2953. doi : 10.1021 / ja01474a037
- ↑ DM Bautista et al .: The menthol receptor TRPM8 is the principal detector of environmental cold. In: Nature . 448, 2007, pp. 204-208.
- ↑ Andreas Bechthold, Robert Fürst, Angelika Vollmar: Biogenic medicinal substances . 1st edition. Wissenschaftliche Verlagsgesellschaft, Stuttgart 2019, ISBN 978-3-8047-3623-8 , pp. 201-202 .
- ↑ BJ Henderson et al .: Menthol Alone Upregulates Midbrain nAChRs, Alters nAChR Subtype Stoichiometry, Alters Dopamine Neuron Firing Frequency, and Prevents Nicotine Reward. In: The Journal of neuroscience: the official journal of the Society for Neuroscience. Volume 36, Number 10, March 2016, pp. 2957-2974, doi : 10.1523 / JNEUROSCI.4194-15.2016 , PMID 26961950 , PMC 4783498 (free full text).
- ↑ K. Ahijevych, BE Garrett: The role of menthol in cigarettes as a reinforcer of smoking behavior. In: Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco. Volume 12 Suppl 2, December 2010, pp. S110-S116, doi : 10.1093 / ntr / ntq203 , PMID 21177367 , PMC 3636955 (free full text) (review).
- ^ RJ Wickham: How Menthol Ages Tobacco-Smoking Behavior: A Biological Perspective. In: The Yale journal of biology and medicine. Volume 88, Number 3, September 2015, pp. 279-287, PMID 26339211 , PMC 4553648 (free full text) (review).
- ↑ L. Biswas, E. Harrison, Y. Gong, R. Avusula, J. Lee, M. Zhang, T. Rousselle, J. Lage, X. Liu: Enhancing effect of menthol on nicotine self-administration in rats. In: Psychopharmacology. Volume 233, number 18, September 2016, pp. 3417–3427, doi : 10.1007 / s00213-016-4391-x , PMID 27473365 , PMC 4990499 (free full text).
- ↑ T. Wang, B. Wang, H. Chen: Menthol facilitates the intravenous self-administration of nicotine in rats. In: Frontiers in behavioral neuroscience. Volume 8, 2014, p. 437, doi : 10.3389 / fnbeh.2014.00437 , PMID 25566005 , PMC 4267270 (free full text).
- ↑ a b Menthol ban from May 20, 2020. www.Tabak-Börse24.de, December 30, 2019, accessed on March 14, 2020 .
- ↑ Appendix 1 , in conjunction with Section 4 Tobacco Products Ordinance (TabakerzV) of April 27, 2016
- ↑ Entry on levomenthol in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on July 19, 2018.
- ↑ FAO Nutrition Meetings Report Series. Vol. 44A, 1967, p. 58.




