Metoprolol
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| Structural formula without stereoisomerism | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Metoprolol | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 15 H 25 NO 3 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action |
selective blockade of β 1 receptors |
|||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 267.36 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
121–124 ° C (tartrate) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Metoprolol is a drug from the group of selective β 1 -adrenoreceptor blockers ( beta blockers ) and is used for the treatment of high blood pressure , coronary artery disease , cardiac arrhythmias and for acute treatment of myocardial infarction . Another area of application is migraine prophylaxis .
Clinical information
Application areas (indications)
Metoprolol can be used in the therapy of myocardial infarction , high blood pressure , coronary artery disease , heart failure and certain cardiac arrhythmias . In addition, metoprolol is used for seizure prophylaxis in migraineurs if certain criteria are met.
Contraindications (contraindications)
Metoprolol may u. a. not in case of decompensated heart failure ( NYHA IV), AV block of the 2nd or 3rd degree, bradycardia (resting pulse less than 50 beats per minute before the start of treatment), hypotension (systolic blood pressure less than 90 mm Hg) and bronchial hyperreactivity ( e.g. in connection with bronchial asthma ) be applied. In patients with peripheral circulatory disorders, the symptoms may intensify.
Drug interactions
The intravenous administration of metoprolol in patients who are already receiving therapy with calcium antagonists (of the verapamil and diltiazem types) or other antiarrhythmics (such as disopyramide ) can lead to severe bradycardiac arrhythmias and is therefore contraindicated; likewise the iv administration of the above-mentioned calcium antagonists and antiarrhythmics under therapy with metoprolol (exception intensive care medicine).
Use during pregnancy and breastfeeding
Metoprolol should only be used during pregnancy and breastfeeding under strict medical supervision. In animal experiments (mouse and rat) there were indications of reduced blood flow to the placenta and, as a result, fetal growth disorders . The risk of cardiac arrhythmias in children cannot be ruled out either.
Special patient groups (diabetics, kidney patients)
If metoprolol and insulin or oral antidiabetic agents are used at the same time, their effect can be increased or prolonged, which increases the risk of hypoglycaemia . At the same time, warning signs of hypoglycaemia ( racing heart and muscle tremors ) are masked or reduced. Therefore, diabetics who are being treated with metoprolol require regular blood sugar controls.
Adverse effects (side effects)
In addition to occasional hypersensitivity reactions, side effects such as ringing in the ears and dizziness caused by the lowering of blood pressure can be observed. Changes in the central nervous system (fatigue, hallucinations), bronchospasm , erectile dysfunction and urination disorders occur less frequently. The ability to actively drive, use machines or work without a secure footing can be impaired by metoprolol. A change in the ability to react is to be expected, especially when starting treatment, increasing the dose or consuming additional alcohol. In predisposed persons, reactions to allergens can be more severe to anaphylactic reaction ( anaphylactic shock ) under beta blocker therapy . MAO inhibitors prevent the breakdown of metoprolol in the organism and thus lead to its accumulation. In addition, metoprolol increases the effects of blood sugar lowering drugs such as insulin and sulfonylureas . When using substances that lower blood sugar, metoprolol can mask the warning signs of hypoglycaemia , especially tremor and tachycardia . Metoprolol also increases the effects of other antihypertensive drugs (indication of the severe drop in blood pressure when β-blockers are used together with calcium antagonists of the verapamil and diltiazem type). Effect-enhancing effects with other drugs that influence heart rhythm are also known. Beta blockers can activate psoriasis in individual cases .
The extent of the symptoms of intoxication in the event of an overdose depends on the amount of substance administered and manifests itself in the form of severe drops in blood pressure, low heart rate and cardiac arrest with corresponding functional failures of the organs. Overdose requires intensive medical treatment and monitoring.
Pharmacological properties
Mechanism of action
Metoprolol blocks β-adrenoceptors , especially β 1 -adrenoceptors , which are mainly located in the stimulation- generating and conduction tissue of the heart (sinus nodes, atria, AV nodes, ventricular muscles ) and in the coronary vessels. As a result, Metoprolol lowers the conduction velocity, the beat frequency and the contraction force of the heart. β 2 -adrenoceptors are not located in the heart tissue, but in the bronchial system , the muscle vessels and other organs such as the urinary bladder, liver and muscles and are only slightly blocked by metoprolol or only blocked at a higher dose. It follows that metoprolol is more likely to be used in patients with chronic respiratory diseases (e.g. with bronchial asthma ) or with peripheral circulatory disorders than β-blockers, which block both β 1 and β 2 adrenoceptors unselectively. The range of side effects can partly be explained by the distribution of the receptors.
Metoprolol does not stimulate the β-adrenoceptors (no intrinsic sympathomimetic activity , ISA) and has only weak membrane-stabilizing properties. The relative potency of metoprolol to propranolol is 1.
After prolonged use, metoprolol intake must not be interrupted or the drug discontinued entirely. The dose must be slowly reduced (treatment tapered off ) in order to avoid excessive circulatory reactions (tachycardia, hypertension) as a rebound effect.
Absorption and distribution in the body
After oral administration, metoprolol is almost completely absorbed in the gastrointestinal tract (around 95%) and is mainly oxidatively metabolized in the liver by the CYP 2D6 isoenzyme . Two of the three main metabolites also show weak beta-blocking properties, but are clinically irrelevant. In the case of liver cirrhosis, due to the reduced metabolism rate, increased plasma levels of unchanged metoprolol must be expected.
Due to the high first-pass effect , around 50% of the original dose is available systemically . The maximum plasma levels are reached after 1.5 to 2 hours. The plasma protein binding is 12% and the relative distribution volume of 5.6 l / kg. About 95% of metoprolol and its metabolites are eliminated renally . The plasma half-life is 3 to 5 hours.
Other Information
Chemical information
Practically all β-receptor blockers have structural features in common. This includes the propanolamine side chain with an isopropyl or tertiary butyl substituent on the nitrogen. The aliphatic hydroxyl group is essential for the beta-blocking effect.
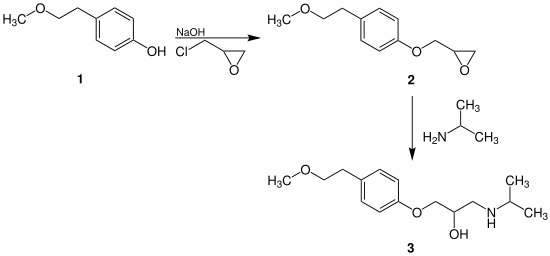
synthesis
Metoprolol ( 3 ) can be synthesized in a two-step reaction:
The phenol derivative 1 reacts with the addition of sodium hydroxide in a nucleophilic substitution with 2- (chloromethyl) oxirane to form epoxide 2 . This then forms the racemic active ingredient metoprolol ( 3 ) with isopropylamine .
Stereoisomerism
Metoprolol is chiral , the active stereoisomer ( eutomer ) is the ( S ) -form of metoprolol. The racemate is used medicinally , with the enantiomers of active ingredients usually having different pharmacological properties and effects. The table shows both stereoisomers. They differ in the position of the hydrogen atom, which in this representation either protrudes from the plane of the drawing ( S ) shape or goes into the plane of the drawing ( R ) shape:
| Enantiomers of metoprolol | |
|---|---|
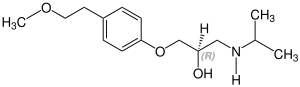 CAS number: 81024-43-3 |
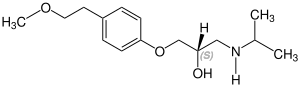 CAS number: 81024-42-2 |
Dosage forms
The active ingredient metoprolol is offered as metoprolol succinate (salt of succinic acid ), as metoprolol tartrate (salt of tartaric acid ) or as metoprolol fumarate (salt of fumaric acid ) in the form of tablets or prolonged-release tablets in various strengths; either as a single preparation or in combination with the diuretic hydrochlorothiazide . A preparation for intravenous use with 5 mg metoprolol tartrate per 5 ml solution for injection is also available.
The sustained release formulations are characterized in part by special release kinetics : the active ingredient is released at a constant rate, which should lead to particularly uniform plasma levels. These dosage forms are usually identified by suffixes such as ZOK, NOK or Zero (for zero order kinetics ).
Market importance
With around 900 million average daily doses , metoprolol was the most widely used beta blocker in Germany in 2012. This makes metoprolol the leading representative of the selective β 1 -adrenoreceptor blockers. The prescription of these has increased by almost 50% in the last 10 years. The non-selective substances, however, are on the decline.
Environmental relevance
Up to 10 percent of the metoprolol taken is excreted unchanged by the body. In this way, the substance reaches surface waters such as rivers via sewage treatment plants , where it is hardly or not at all degraded. Concentrations in the two to four-digit nanogram per liter range are detected there, which means that metoprolol is often found in the highest environmental concentrations among the beta blockers examined.
Trade names
Monopreparations
- Beloc (D, CH), Beloc-ZOK (CH), Beloc-ZOK Herz (D), Lopresor (D, CH), Metopress (CH), Seloken (A), numerous generics
Combination preparations
- with nifedipine : Belnif (D)
- with hydrochlorothiazide : Beloc-ZOK comp (D)
- with felodipine : Mobloc (D), Logimax (CH),
- with chlorthalidone : Prelis comp. (D), Logroton (CH)
literature
- Claus-Jürgen Estler: Pharmacology and Toxicology . Schattauer Verlag, 1999, ISBN 3-7945-1895-0 .
- E. Oberdisse, E. Hackenthal, K. Kuschinsky (Eds.): Pharmacology and Toxicology. Springer Verlag, 1997, ISBN 3-540-61953-4 .
Web links
- MedlinePlus Druginfo (English)
Individual evidence
- ↑ Data sheet METOPROLOL TARTRATE CRS (PDF) at EDQM , accessed on July 14, 2008.
- ↑ Data sheet (±) -Metoprolol (+) - tartrate salt from Sigma-Aldrich , accessed on October 22, 2016 ( PDF ).
- ↑ Entry on metoprolol in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Entry on metoprolol. In: Römpp Online . Georg Thieme Verlag, accessed on December 25, 2014.
- ↑ Axel Kleemann , Jürgen Engel, Bernhard Kutscher, Dietmar Reichert: Pharmaceutical Substances - Syntheses, Patents and Applications of the most relevant APIs. 5th edition. Georg Thieme Verlag, 2009, ISBN 978-3-13-558405-8 , pp. 883-884.
- ↑ Joni Agustiana, Azlina Harun Kamaruddina, Subhash Bhatiaa: Single enantiomeric blockers — The existing technologies. In: Process Biochemistry. 45, 2010, pp. 1587-1604.
- ^ EJ Ariëns: Stereochemistry, a basis for sophisticated nonsense in pharmacokinetics and clinical pharmacology. In: Eur J Clin Pharmacol . 26 (2), 1984, pp. 663-668. PMID 6092093 .
- ↑ Rote Liste Service GmbH (Ed.): Rote Liste 2017 - drug directory for Germany (including EU approvals and certain medical devices) . Rote Liste Service GmbH, Frankfurt / Main, 2017, edition 57, ISBN 978-3-946057-10-9 , p. 200.
- ↑ Dieter Paffrath, Ulrich Schwabe: Drug Ordinance Report 2013. Springer, 2013, ISBN 978-3-642-37123-3 , p. 478.
- ^ A b Thomas A. Ternes: Occurrence of drugs in German sewage treatment plants and rivers. In: Water Research . 1998, doi: 10.1016 / S0043-1354 (98) 00099-2 .
- ↑ Meritxell Gros, Mira Petrović, Antoni Ginebreda, Damià Barceló: Removal of pharmaceuticals during wastewater treatment and environmental risk assessment using hazard indexes. In: Environment International . 2010, doi: 10.1016 / j.envint.2009.09.002 .
- ↑ Wibke Meyer, Margrit Reich, Silvio Beier, Joachim Behrendt, Holger Gulyas, Ralf Otterpohl: Measured and predicted environmental concentrations of carbamazepine, diclofenac, and metoprolol in small and medium rivers in northern Germany. In: Environmental Monitoring and Assessment. 2016, doi: 10.1007 / s10661-016-5481-2 .
- ^ Andreas Fath: Rhine water - 1231 kilometers with the current. Carl Hanser Verlag, Munich 2016, ISBN 978-3-446-44871-1 , p. 135.