N, N-dimethylaminododecane
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | N, N -dimethylaminododecane | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | C 14 H 31 N | ||||||||||||||||||
| Brief description |
clear, colorless liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 213.40 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.787 g cm −3 at 25 ° C |
||||||||||||||||||
| Melting point |
- 20 ° C |
||||||||||||||||||
| boiling point | |||||||||||||||||||
| Vapor pressure |
0.012 hPa at 20 ° C |
||||||||||||||||||
| solubility |
practically insoluble in water, soluble in chloroform and isopropanol , miscible with organic solvents |
||||||||||||||||||
| Refractive index |
1.4354 (25 ° C, 589 nm) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Dodecyldimethylamine ( DDA ) is a tertiary fatty amine with a linear C 12 ( dodecyl ) carbon chain and two methyl groups on the nitrogen atom. The on lauric acid particularly rich (lauric acid) plant oils coconut oil and palm oil are renewable raw materials for the oleochemical synthesis of lauryldimethylamine, which in turn important starting material for surfactants surfactants and antibacterial effective quat are.
Occurrence and representation
Dodecyldimethylamine is not a naturally occurring compound, but can be obtained from functional n - dodecane derivatives in different ways on a laboratory and industrial scale. Syntheses from dodecyl bromide and dimethylamine or dodecylamine and dimethyl sulfate are more suitable for small quantities, while the Eschweiler-Clarke methylation of dodecylamine with formic acid - formaldehyde mixture supplies N, N -dimethylaminododecane with a yield of approx. 90%.
The primary amine laurylamine can also be reacted with formalin and hydrogen on a nickel contact to form N, N- dimethyldodecylamine (93.6% yield).
Dodecanol reacts with dimethylamine and hydrogen on a copper chromite contact to form DDA in 95% yield.
For industrial production, synthesis routes are relevant that start directly from the fractionated saponification products of vegetable oils, i.e. H. start from lauric acid or lauric acid methyl ester . Direct amidation of lauric acid with dimethylamine initially yields the salt dimethylammonium laurate, which converts to N, N- dimethyldodecanamide by splitting off water when heated .
Instead of lauric acid, the methyl ester can also be used, as is obtained in biodiesel production by transesterification of the vegetable oils with methanol and separation by distillation as pure methyl laurate (or a C 12 / C 14 mixture rich in lauric acid). The carboxamide can be obtained from the ester in yields> 90% with dimethylamine and sodium methoxide as catalyst at significantly lower temperatures (approx. 100 ° C) than from the carboxylic acid. Another advantage of conducting the process at the lowest possible temperatures is the reduction or avoidance of yellowing and, in particular, odor formation.
The N, N -dimethyldodecanamide can be hydrogenated practically quantitatively to DDA on nickel or industrially on copper chromite contacts.
properties
N, N -dimethylaminododecane is a colorless liquid with an ammoniacal-fish-like odor, which mainly comes from contamination with triethylamine , which can be removed practically completely by heating in a vacuum or passing nitrogen through.
Applications
The amine dodecyldimethylamine and its salts with mineral acids, such as. B. phosphoric acid or sulfuric acid are used as corrosion inhibitors , asphalt emulsifiers, flotation aids , additives for cooling lubricants and drilling fluids . The free amine is the starting material for surface-active compounds with different functional (cationic, nonionic and amphoteric) head groups, such as. B.
Quats from DDA
Quaternization of dodecyl to as Quat designated Dodecyltrimethylammoniumsalz can with alkylating agents such. B. methyl chloride , methyl bromide , dimethyl sulfate or dimethyl carbonate can be carried out.
The reaction with excess gaseous methyl chloride takes place in an autoclave at an elevated temperature, the quaternary ammonium chloride being formed in quantitative yield as a colorless powder. The reaction in the other, liquid alkylating agents gives the corresponding C 12 ammonium salts, which are readily water-soluble, but mostly hygroscopic. Dodecyltrimethylammonium chloride (CAS # 112-00-5) is used as a foam stabilizer , phase transfer catalyst , antistatic and especially bacteriostatic .
Amine oxides from DDA
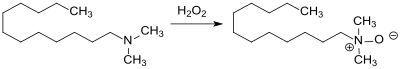
With 35% hydrogen peroxide , N, N- dimethylaminododecane is oxidized to the amine oxide dodecyldimethylamine oxide, which has surface-active properties and is used in shampoos and cleaning agents because of its synergistic effects with anionic and nonionic surfactants.
Betaines from DDA
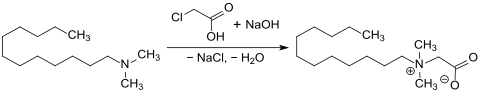
Carboxymethylation of dodecyldimethylamine with monochloroacetic acid MCA or sodium monochloroacetate SMCA in an ethanol / water mixture 1: 1 provides the amphoteric surfactant dodecyldimethylbetaine (laurylbetaine) (CAS # 683-10-3), which is used as a foam stabilizer in baby shampoos because of its low skin and eye irritation and enhancers, as dispersants , e.g. B. is used for pesticides , as well as thickening and wetting agents.
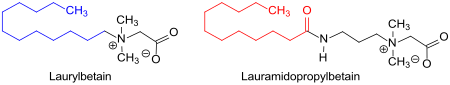
Lauryl betaine - as a derivative of the amino acid glycine , or betaine , in which a methylene group by a C 12 - alkyl group is replaced (blue) - not to be confused with lauramidopropyl betaine , wherein the C 12 - acyl group (red) comes from the lauric acid. The amidopropyl betaine is obtained by reaction of lauric acid with 3-aminopropyldimethylamine to form the amidoamine and subsequent carboxymethylation with MCA.
Sulfobetaines from DDA
Sultones , such as B. propane react with lauryldimethylamine the corresponding sulfobetaine Laurylsulfobetain (CAS # 14933-08-5), the membrane proteins largely without denaturation solubilized .
While the carboxylate group of the amino acid derivative lauryl betaine protonates to the carboxy group in acid (pH <5) and the betaine becomes a cationic surfactant , the corresponding sulfobetaine remains completely dissociated even at a strongly acidic pH , i.e. it retains its zwitterionic character.
Hydroxysultaine from DDA
An additional hydroxyl group and thus an even more hydrophilic head group than sulfobetaines have hydroxysultaines, as they are, for. B. in the reaction of the sodium salt of 1-chloro-2-hydroxypropanesulfonic acid CHPS (by addition of sodium hydrogen sulfite NaHSO 3 to epichlorohydrin ) with N, N -dimethyldodecylamine to lauryl hydroxysultaine (CAS # 13197-76-7).
Hydroxysultaines are neutral and very mild amphoteric surfactants in water, which foam strongly even in hard water or salt water and are stable over a wide pH range. They are well tolerated and biodegradable with all tenside classes. As so-called co-surfactants, hydroxysultaines allow less use of standard surfactants and, because of their viscosity-increasing effect, avoid the addition of sodium chloride.
Manufacturers and suppliers
N, N -Dimethylaminododecane is supplied by Albemarle Corporation (adma® 12), Eastman Chemical (Dimla ™ 12), Global Amines (a joint venture between Clariant AG and Wilmar International ) (Genamin® 12 R302 D), Kao Corporation (FARMIN® DM2098) , Solvay SA (Fentamine® Dma12) and some Asian companies.
Individual evidence
- ↑ Entry on DIMETHYL LAURAMINE in the CosIng database of the EU Commission, accessed on June 25, 2020.
- ↑ a b Entry on dodecyldimethylamine in the GESTIS substance database of the IFA , accessed on June 18, 2020 (JavaScript required)
- ↑ a b c d e f g data sheet N, N-dimethyldodecylamine from Sigma-Aldrich , accessed on June 14, 2020 ( PDF ).
- ^ A b Carl L. Yaws: Thermophysical Properties of Chemicals and Hydrocarbons, 2nd Edition . Elsevier Inc., Oxford, UK 2015, ISBN 978-0-323-28659-6 , pp. 475 .
- ↑ Technical Data Sheet: DIMLA TM 1214. Eastman Chemical Co., August 21, 2019, accessed June 18, 2020 .
- ↑ Patent US2366534 : Production of long-chain tertiary amines. Filed July 30, 1941 , published January 2, 1945 , applicant: EI du Pont de Nemours & Co., inventor: JE Kirby.
- ↑ Patent EP0323573A2 : Process for the preparation of tertiary N, N-dimethylamines. Applied on November 30, 1988 , published on July 12, 1989 , applicant: Hoechst AG, inventor: J. Weber, D. Kampmann, C. Kniep.
- ↑ Patent US7402702B2 : Process for producing tertiary amines. Filed November 30, 2005 , published July 22, 2008 , applicant: Kao Corp., inventor: S. Oguri, T. Nishimura.
- ↑ RM Lanigan, TD Sheppard: Recent developments in amide synthesis: Direct amidation of carboxylic acids and transamidation reactions . In: Eur. J. Org. Chem. Volume 33 , 2013, p. 7453-7465 , doi : 10.1002 / ejoc.201300573 .
- ↑ Patent US3288794 : Method of making amides of dimethylamine and piperazine. Filed September 9, 1963 , published November 29, 1966 , Applicant: The CP Hall Company of Illinois, Inventor: VP Kuceski.
- ↑ J. Ding, L. Chen, R. Shao, J. Wu, Z. Yu, W. Dong: Synthesis of N, N -dimethyldodecylamine by the catalytic hydrogenation of N, N-dimethyldodecylamide . In: REACT KINET MECH CAT . tape 108 , no. 1 , 2013, p. 151-159 , doi : 10.1007 / s11144-012-0505-6 .
- ↑ Patent EP1736463B1 : Process for obtaining amines by reduction of amides. Registered on June 22, 2005 , published on October 5, 2011 , applicant: Taminco NV, inventors: R. Loenders, I. van den Eynde, P. Vanneste.
- ↑ Patent EP0723952B2 : Process for the purification of tertiary fatty alkylmethylamines. Registered on January 20, 1996 , published on September 20, 2000 , applicant: Clariant GmbH, inventor: B. Papenfuhs, H. Seitz, A. Gallus.
- ↑ Patent US3308161 : Alkali metal-dimethyl dodecyl amine salts of oxyacids of phosphorous and sulfur. Applied on May 21, 1962 , published March 7, 1967 , applicant: Petrolite Corp., inventor: K.-T. Shen.
- ↑ Patent DE3713996A1 : Process for the production of quaternary ammonium halides . Applied on April 27, 1987 , published on November 10, 1988 , Applicant: Henkel KGaA, Inventors: H. Rutzen, P. Lorenz, H. Tesmann.
- ↑ Patent DE19528945A1 : Process for the production of tertiary amine oxides. Applied on August 7, 1995 , published on February 13, 1997 , applicant: Hoechst AG, inventor: G. Baeder, RJ Tamarg.
- ↑ BETADET® S-20. In: Technical Data Sheet. Kao Corporation, SA, accessed June 20, 2020 .