2,3,7,8-tetrachlorodibenzodioxin
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 2,3,7,8-tetrachlorodibenzodioxin | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 12 H 4 Cl 4 O 2 | |||||||||||||||
| Brief description |
colorless crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 321.97 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.83 g cm −3 |
|||||||||||||||
| Melting point |
295 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
0.01 ng m −3 |
|||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
2,3,7,8-Tetrachlorodibenzodioxin is a chlorine-containing , highly toxic organic compound . The systematic name is 2,3,7,8-tetrachlorodibenzo- p- dioxin or 2,3,7,8-tetrachlorodibenzo-1,4-dioxin (pronounced two- three- seven- eight- tetrachlorodibenzopara- di-oxine ). The substance is derived from dibenzodioxin and is abbreviated as 2,3,7,8-TCDD or just TCDD , colloquially often (incorrectly) referred to as dioxin or Seveso- dioxin or Seveso poison . The short name dioxin often unspecifically describes the entire superordinate group of substances of polychlorinated dioxins and dibenzofurans , the most toxic representative of which is 2,3,7,8-tetrachlorodibenzodioxin.
TCDD can arise as an undesirable by-product in the production of the economically very important organochlorine compounds ( chlorine chemistry ) and in the incineration of similar substances. Typical concentrations are in the ppm range; in isolated, serious chemical accidents such as the Italian Sevesoung accident in 1976, quantities up to the kilogram range were produced. Traces of TCDD can also arise in forest fires ; other sources are engine exhaust fumes , cigarette smoke , fireworks and, in general, smoldering processes at low temperatures. The substance is a poison or pollutant and has no technical or economic usefulness. Standard analytical methods for the detection and evaluation of polyhalogenated dibenzodioxins and -furans were mainly worked out by Karlheinz Ballschmiter .
history
TCDD was first synthesized in 1957 by Wilhelm Sandermann in the laboratory. He also discovered the effects of TCDD.
The defoliant Agent Orange was used in the Vietnam War from 1965 to 1970 , the contamination of which with TCDD led to severe damage to the population and US soldiers, which continues to this day.
On 10 July 1976, it came to the devastating Seveso disaster in which in the northern Italian town of Seveso leaked from a few hundred grams and a few kilograms of TCDD.
During the 2004 presidential elections in Ukraine , suspicions of dioxin poisoning were confirmed when candidate Viktor Yushchenko was found in his blood and tissues to be more than 50,000 times the normal concentration of TCDD. Yushchenko's face has been showing severe symptoms of chloracne since the alleged attack .
Occurrence and discovery of its importance as a toxin
TCDD is formed together with other polychlorinated dibenzodioxins and dibenzofurans as a by-product in the synthesis of the economically very important organic chlorine compounds ( e.g. halophenoxycarboxylic acid herbicides) or the combustion of compounds containing chlorine and hydrocarbons. The undesired synthesis of dioxins can be almost completely avoided by appropriate design and control of the chemical manufacturing processes, in particular the reaction temperatures used. However, in the first decades of industrial production and use of this class of substances, this was hardly researched or known, as dioxin was only discovered in 1957 and knowledge of its undesirable formation and high toxicity only gradually arose due to the occurrence of corresponding health damage.
Because of the extreme increase in importance of organic chlorine compounds in the second half of the 20th century - especially in crop protection , plastics and flame retardant production - the problem of the undesired formation of dioxins in such production processes became increasingly important from around the 1950s. Initially, this was shown above all in the increased health damage suffered by workers in the chemical industry, such as (among other things) dioxin-induced chloracne , although the search for the cause of the serious health damage was initially in vain.
While the so-called "chlorine chemistry" has largely resolved the dioxin problem through adaptation of industrial production methods and processes, waste incineration plants are still considered a potential source of dioxins - there it is created in the presence of chlorine-containing compounds such as PVC during incineration. Modern incinerators therefore reheat to over 1200 ° C with subsequent rapid cooling, which reduces the TCDD concentration to a fraction.
properties
2,3,7,8-Tetrachlorodibenzodioxin is a long-lived, very toxic pollutant that is in crystalline form at room temperature. In addition, it is readily soluble in organic solvents and lipophilic (readily soluble in fat and oil), which is why it also accumulates in human adipose tissue . The logarithmic octanol-water partition coefficient log K OW of TCDD is 6.80.
use
TCDD, like all other dioxins and furans, is an undesirable by-product with no economic benefit. There is currently no commercial use for this group of substances.
In animal research, it is used as an agonist for the aryl hydrocarbon receptor ( Ah receptor for short ).
Conditions and mechanism of formation
Dioxins are not specifically produced, except for the purposes of research and chemical analysis . They arise as by-products in a large number of thermal processes in the presence of certain starting materials. The TCDD treated here is usually created as a mixture of substances together with other dioxins, i.e. substances that have a different number of chlorine atoms (than four in the TCDD) also in other positions (than 2,3,7,8) of the dibenzodioxin skeleton contain.
When organic (carbon-containing) compounds are burned in the presence of organic or inorganic halogen compounds (especially chlorine or bromine ), they can form in a temperature range of around 300-600 ° C, which is known as the dioxin window. Incineration processes with possible dioxin formation are, for example, cremation in crematoria and waste incineration , which was one of the main causes of dioxin production until the 1980s. Since stricter limit values were introduced in Germany in 1990, dioxin pollution from waste incineration plants and cremation has fallen to practically zero today. Other industrial processes that can produce dioxins include:
- Bleaching processes with chlorine in papermaking
- the production of pesticides
- metallurgical processes (e.g. iron and steel production)
- Production of chlorophenols .
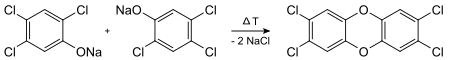
The heat effect on polychlorinated, i.e. multiply chlorinated, phenols is particularly critical . These condense particularly easily in the presence of alkali via the phenolates to dioxin. This is how 2,3,7,8-TCDD is formed from the sodium salt of 2,4,5-trichlorophenol (2,4,5-TCP):
Trichlorophenol (TCP, in the graphic below in the middle) was produced in the Icmesa chemical plant in Seveso , Italy , where the most famous chemical accident with massive TCDD release took place in 1976, the so-called Sevesoung accident . This served as a preliminary product for the disinfectant hexachlorophene . TCP was created from the raw material tetrachlorobenzene (left) by adding alkaline sodium hydroxide (NaOH):
This reaction produces 2,3,7,8-tetrachlorodibenzodioxin (right) as a by-product, especially at elevated temperatures. The accident happened in a reaction vessel ( autoclave ), the temperature of which was no longer monitored after the end of a production cycle. If the mechanical agitator for the vessel was improperly switched off, a subsequent heat build-up resulted. This led to the formation of large amounts of TCDD and a sharp rise in pressure, whereupon a safety valve opened and a considerable amount of the high-dioxin mixture was blown directly into the inhabited area of the factory.
Effects dangerous to health
The lethal dose in humans is not known; in animal experiments the lethal dose for oral administration was between 0.5 ( guinea pigs ) and 1157 µg / kg body weight ( hamsters ), depending on the species . Contact with TCDD can lead to the appearance of chloracne , which is a symptom of severe dioxin poisoning. Such poisoning can also lead to severe organ damage , especially the liver . A potentially mutagenic effect, i.e. damage to the genetic make-up , has not been clearly demonstrated. The question of whether dioxins can cause malformations in the offspring ( teratogenic effect) cannot be answered with certainty either.
In Vietnam and the USA, multiple teratogenic malformations were found in the descendants of people contaminated with Agent Orange - which, in addition to 2,4,5-T as a herbicide, also contained TCDD contaminants. A meta-study that was published in the International Journal of Epidemiology in 2006 and evaluated 22 studies from 1966 to 2002 came to the result: Parental exposure to Agent Orange appears to be associated with an increased risk of birth defects.
It is also considered certain that dioxins can cause cancer . It has not been conclusively clarified whether TCDD has a direct carcinogenic effect or acts as a tumor promoter .
According to current knowledge, the toxic effect of TCDD is mediated by activating the Ah receptor , which - like the receptors for steroid hormones and thyroid hormones - can bind to certain regulatory DNA sequences and thereby regulate the expression of various genes . ITE is assumed to be the natural ligand of the Ah receptor (see figure). The TCDD-mediated activation of the Ah receptor leads, among other things, to a strong induction of cytochrome P450 .
Metabolites
In toxicokinetic studies on the poisoning of Ukrainian presidential candidate Viktor Yushchenko two were metabolites in serum, urine and feces demonstrated. These are 2,3,7-trichloro-8-hydroxydibenzo- p- dioxin and 1,3,7,8-tetrachloro-2-hydroxydibenzo- p- dioxin. The feces were identified as the main route of elimination from the human organism. The detection was carried out by coupling the capillary gas chromatography with the mass spectrometry , as is usual with the analysis of the PCDD .
Individual evidence
- ↑ a b c d e Entry on 2,3,7,8-tetrachlorodibenzodioxin in the GESTIS substance database of the IFA , accessed on December 19, 2008. (JavaScript required)
- ^ Mary Lide, David R. Lide: CRC Handbook of Chemistry and Physics . 87th edition, CRC Press, 2007, ISBN 978-0-8493-0594-8 , p. 470.
- ↑ a b Entry on 2,3,7,8-tetrachlorodibenzo [1,4] dioxin. In: Römpp Online . Georg Thieme Verlag, accessed on May 24, 2014.
- ↑ There is not yet a harmonized classification for this substance . A labeling of 2,3,7,8-tetrachlorodibenzo [b, e] [1,4] dioxin in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on April 29, 2015, is reproduced from a self-classification by the distributor .
- ↑ a b c d e f g Entry on 2,3,7,8-tetrachlorodibenzo-p-dioxin in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on August 8, 2016.
- ^ A b c Wilhelm Sandermann: Dioxin. The history of the discovery of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD, Dioxin, Sevesogift) ( Memento of December 14, 2008 in the Internet Archive ) 1984, accessed on August 13, 2012.
- ^ Biography of Wilhelm Sandermann ( Memento from September 27, 2007 in the Internet Archive ).
- ↑ PA Bertazzi, I. Bernucci, G. Brambilla, Consonni D., AC Pesatori: The Seveso studies on early and long-term effects of dioxin exposure: a review . In: Environmental Health Perspectives . 106, April 1998, pp. 625-633. doi : 10.1289 / ehp.98106625 .
- ↑ a b O. Sorg, M. Zennegg, P. Schmid, R. Fedosyuk, R. Valikhnovskyi, O. Gaide, V. Kniazevych, J.-H. Saurate: 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) poisoning in Victor Yushchenko: identification and measurement of TCDD metabolites . In: The Lancet . tape 374 , no. 9696 , 2009, p. 1179–1185 , doi : 10.1016 / S0140-6736 (09) 60912-0 , PMID 19660807 .
- ^ Ottfried Strubelt: Poisons in nature and the environment. Pesticides and heavy metals, pharmaceuticals and drugs . Spectrum Academic Publishing House, Heidelberg / Berlin / Oxford 1996, pp. 183–192.
- ^ Rene P. Schwarzenbach , Philip M. Gschwend, Dieter M. Imboden: Environmental Organic Chemistry . Wiley-Interscience, Hoboken, New Jersey 2003, ISBN 0-471-35750-2 .
- ^ Gordon McKay: Dioxin characterization, formation and minimization during municipal solid waste (MSW) incineration: review . In: Chemical Engineering Journal . tape 86 , no. 3 , 2002, p. 343-368 , doi : 10.1016 / S1385-8947 (01) 00228-5 .
- ↑ PA Bertazzi, I. Bernucci, G. Brambilla, Consonni D., AC Pesatori: The Seveso studies on early and long-term effects of dioxin exposure: a review. In: Environmental Health Perspectives. 106 (Suppl 2), 1998, pp. 625-633, PMC 1533388 (free full text).
- ↑ “I was absolutely stupid” ( taz interview with Jörg Sambeth, July 10, 2006).
- ↑ H. Künzi: Thermal safety investigations of a sodium 2,4,5-trichlorophenolate reaction mixture , in Chimia 36 (1982), 162-168.
- ↑ Anh D. Ngo, Richard Taylor, Christine L. Roberts, Tuan V. Nguyen: Association between Agent Orange and Birth Defects: Systematic Review and Meta-Analysis . In: International Journal of Epidemiology . tape 35 , no. 5 , 2006, p. 1220-1230 , doi : 10.1093 / ije / dyl038 , PMID 16543362 .