Tobacco smoke
Tobacco smoke arises from the fire or smoldering of tobacco z. B. in cigarettes , cigars , pipes or water pipes . It is an aerosol made from smoke , vapors , gases and solids . A distinction is the mainstream smoke that the smoker inhales directly, and the sidestream smoke of burning tobacco goods.
Tobacco smoke contains a variety of combustion products. The tobacco smoke produced when smoking a cigarette contains all organic compounds in various forms of oxidation and decay that arise from the smoked tobacco and possibly the wrapping and do not remain in the ash. The analysis can identify up to 9600 different chemical compounds in different proportions. According to a publication by the International Agency for Research on Cancer (IARC) of the World Health Organization (WHO), a total of 69 chemical compounds classified as carcinogenic had been identified in tobacco smoke by the year 2000. With regard to their physiological effect, these compounds can be divided into five groups.
- carcinogenic (carcinogenic) substances,
- (blood) toxic substances,
- neurotoxic substances,
- irritating substances ,
- physiologically harmless substances.
Chemistry of tobacco smoke
The smoke aerosol is a mixture of
- solid-liquid particles with a phase fraction of five to ten percent, a particle diameter of 0.1 micrometer to 1.0 micrometer and a particle concentration of 10 7 -10 10 particles per milliliter of smoke,
- Vapors, that is the condensable gas vapor phase from vaporized liquids and from
- the gas phase.
The (smoke) condensate (smoke deposits, agglomeration of solid-liquid particles) is colloquially also called "tar". The average condensate content of a single German-made cigarette was 9 to 25 milligrams in 1975 and 12 to 14 milligrams in 1990.
The chemical composition of tobacco smoke has to be assessed differently depending on the air flow in which the tobacco smoke is generated. Main and sidestream smoke (in the English-language specialist literature environmental tobacco smoke , ETS) are composed of six different currents.
- Combustion current ( glow stream ): It is released from the embers on the cigarette holder when you draw.
- Carbonization stream ( side stream ): It is released by the embers of the cigarette holder during the pause.
- Smoulder stream : The one that is emitted in the mouthpiece area during pauses between the draws.
- Diffusion flow: The portion that passes from the inside of the cigarette through the filter paper during the pause.
- Effusion current: Corresponding to the portion that comes from the inside of the cigarette when it is pulled through the filter paper.
- Exhalation stream ( blow stream ): The proportion of smoke exhaled by the smoker, whereby most of the pollutants are filtered out in the lungs and usually remain there.
Three different chemical processes take place in tobacco smoking.
- The tobacco burns through the redox reaction in the glowing zone when air is drawn through it. Cigarettes reach a temperature of 800 ° C to 1100 ° C, cigars from 580 ° C to 660 ° C and when smoking a pipe the tobacco burns up at 420 ° C to 500 ° C in an oxidizing atmosphere.
- The tobacco smoldered behind the glowing cone and inside the glowing cone in a reducing atmosphere. This causes incomplete combustion and thermal decomposition ( pyrolysis ). This carbonization releases many harmful, “unsaturated” compounds, condensation and polymerization products . Depending on the distance from the glowing zone, it takes place at 200–600 ° C.
- Volatile components contained in the tobacco evaporate and distill in an evaporation and distillation zone behind this smoldering zone. Low-boiling substances go directly into the smoke. The steam released when smoking takes pollutants with it through steam distillation and extraction , and so nicotine and essential oils get into the smoke.
Pollutants in tobacco smoke
In addition to well-known pollutants such as benzene , hydrogen cyanide , formaldehyde and nitrosamines , the aerosol of cigarette smoke contains other substances with different hazard potential.
Substances toxic to blood
First and foremost, carbon monoxide (CO) should be mentioned. It is a colorless, odorless gas with a high toxicity ( toxicity ) as hydrogen cyanide gas . It is formed in traces when the tobacco is not completely burned. When inhaled and absorbed through the lungs, it binds to the red blood pigment hemoglobin . Since CO is bound more tightly than oxygen, the blood's ability to transport oxygen decreases.
However, in contrast to the highly toxic hydrogen sulfide with its rotten egg smell or the hydrogen cyanide gas with its bitter almond smell, CO is odorless, so that the body is not warned if large amounts of carbon monoxide are inhaled. Carbon monoxide poisoning is not possible through smoking tobacco alone (cigarette or cigar), but the ability of the blood to absorb oxygen from the air and transport it through the body is impaired. As a result, a smoker can “run out of breath” or “run out of breath” more than a non-smoker. The situation is different when using the water pipe , as the tobacco smoke contains large amounts of carbon monoxide.
Neurotoxic substances
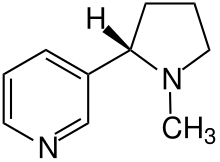
The nicotine (also: nicotine, chemically: α-pyridyl-β- N -methyl-pyrrolidine, C 10 H 14 N 2 ) itself - in addition to the nornicotine and the aromatic agent Nikotianin - the actually effective, psychoactive component of the drug tobacco. As a substance, it is an oily, colorless liquid. In terms of structure, it is a nitrogen base, an alkaloid . Nicotine belongs to the group of drugs and neurotoxins with a narcotic effect. For a long time it was assumed that even if 60 mg of nicotine were swallowed, an adult was in danger of death. This assumption was based on the research results of the toxicologist and pharmacologist Rudolf Kobert. In 1906 he published the textbook of intoxications , in which he relied on experimental results of 2-4 mg and deduced from this that the maximum lethal oral nicotine dose could not be higher than 60 mg. Kobert traced his surveys back to self-experiments by the Austrian doctor Karl Damian von Schroff in 1856. In 2014, the pharmacologist Bernd Mayer from the Karl-Franzens University in Graz corrected the value to over 500 mg.
The absorption of the neurotoxin into the body (intoxication) depends on many factors.
- Method of tobacco use (smoking, chewing, sniffing)
- Inhalation depth
- Inhalation time
- Number of trains per piece
- Stub length, i.e. how much is thrown away in the "dump".
The smoldering of the tobacco destroys around two thirds of the amount of nicotine in the tobacco, so only one third goes into the smoke. As a result, with active and passive smoking, only a fraction of the amount of nicotine inhaled with the smoke gets into the bloodstream via the oral mucous membranes (five percent) and the lungs (the remaining 95 percent). There it initially slows down the pulse and, depending on the type and amount of further nicotine intake, after the start of the drop in blood pressure, it ultimately ensures an increase in the pulse frequency, an increase in bowel movements, a drying up of the glandular secretions in the body and a - more quickly recognizable - pupil constriction in the eye Accommodation spasm. Later, the pupil dilation occurs due to paralysis of the eye muscle. With higher dosages (nicotine intake into the blood), there is a tendency to local and ultimately even life-threatening paralysis of all muscles including the heart. The fatal circulatory collapse is reached from around 500 milligrams.
There have also been cases of young children who have eaten abandoned cigarettes while playing unsupervised, resulting in death. It is believed that the nicotine contained in tobacco is primarily responsible for death.
The pyridine-3-carboxylic acid known as "nicotinic acid" is a component of the vitamin B2 complex, which is used as a food supplement or in medicines. Brewer's yeast contains it as nicotinic acid amide. In contrast to the neurotoxins nicotine, nornicotine and the aromatic active ingredient nicotianine, there is no toxic effect.
Carcinogenic Substances
Tobacco smoke contains a large number of carcinogenic substances. However, these range in very different amounts from toxic heavy metals such as cadmium to blood poisons such as benzene to tar particles and the highly carcinogenic benzopyrene . Some traces of the radioactive heavy metal polonium are contained in tobacco smoke. The substances in tobacco and smoke differ greatly depending on the variety and the phosphate fertilizers used.
As early as 1958, Ernest L. Kennaway and AJ Lindsay identified certain anthracenes , benz perylene , pyrene , chrysene and arsenic as carcinogenic (cancer-causing) substances in tobacco smoke.
Irritating substances
Irritant substances slow down the self-cleaning system of the bronchi , so that a chronic bronchitis (“smoker's cough”) develops with regular tar and condensate supply. Despite quitting smoking completely immediately, these complaints persist for months to years. The irritant substances include nitrogenous compounds such as ammonia (chemical formula: NH 3 ) and nitrogen oxides such as NO and NO 2 and N 2 O 4 . The nitrogen oxides are also suspected of being carcinogenic.
More detailed chemoanalytical information on tobacco smoke components
Condensate values on cigarette packs indicate three analytical values. Only a small fraction of the substances in the smoke determines the tobacco aroma, the main part consists of pollutants, some of which are barely perceptible.
Sidestream smoke can also be burdensome for non-smokers exposed to tobacco smoke. The occupational exposure limit used as a benchmark (formerly MAK value ) is the maximum workplace concentration for which no health damage is to be expected over the working life, based on an eight-hour day and a 5-day week . If this is exceeded, the employer is liable to prosecution in the appropriate circumstances, but in any case acts improperly and can be fined in working life. In toxicology, the lethal dose LD 50 indicates the amount of substance which , if ingested , at least 50 percent of the treated animals die within 24 hours. Tobacco smoke contains the following groups of substances:
- Gas phase
- Water vapor: ten to 20 percent
- Nitrogen N 2 from the air (up to 73%), oxygen O 2 (10%) and carbon dioxide CO 2 as a combustion product (up to 9.5% - normal in the air: 0.04%, AGW 9,000 mg, i.e. 5,000 ml per Cubic meters of breathing air per eight-hour working day)
- the hemoglobin blocker carbon monoxide CO (4.2%, toxic, odorless)
- Hydrogen H 2 (1%)
- Noble gases (Ar, Kr, Ne, Xe: 0.6%)
- Hydrocyanic acid HCN (0.16%, a toxic hemoglobin blocker, 50 mg is lethal within seconds, the MAK value is 11 mg per cubic meter)
- Ammonia NH 3 (0.03%, an irritant and war gas, AGW 15 mg / cubic meter or 20 ml / cubic meter)
- Nitrogen oxides (0.02%, carcinogenic) as well
- Hydrogen sulfide H 2 S (in traces, but also a hemoglobin blocker, highly toxic, MAK value therefore at 15 mg or 10 ml per cubic meter, LC 50 value in rats, inhaled: 0.44 ml / m³)
- Organic compounds
- Formic acid (HCOOH, liquid, a corrosive acid)
- the alcohols methanol (CH 3 OH) and ethanol (C 2 H 5 OH; methanol leads to blindness when consumed orally, LD 50 = 5.6 g / kg, orl, rat, the AGW for ethanol is 500 ml / cubic meter)
- the aldehydes methanal (HCHO, "formaldehyde"), ethanal (acetaldehyde, CH 3 CHO), acrolein (CH 2 = CH-CHO, prop-2-enal), 2-butanone (methanal is a poisonous allergen, MAK value: 0.5 ml / cubic meter, ethanal (acetaldehyde) is poisonous and acrolein (acrylaldehyde is highly toxic and carcinogenic, AGW: 0.09 ml / cubic meter)
- Hydrocarbons of the substance classes of alkanes, alkenes and alkynes (aliphatic) such as ethane, butane, ethene , propene, butene , 1,3-butadiene , ethyne and aromatic hydrocarbons ( benzene (C 6 H 6 ) and toluene (C 7 H 8 )) . Benzene, a highly carcinogenic blood poison, has a MAK value of 1.0–2.5 ml / cubic meter)
- In addition, tobacco smoke and vapor contain: phenol (= hydroxybenzene), as esters alkyl methanoates and ethanoates, as amines also aminomethane and ethane, dimethylamine (aminomethane is methylamine: AGW = 10 ml / cubic meter)
- Particle Ingredients
- Overall, the particle content in tobacco smoke includes substances from the substance classes listed below, some of which are toxic . The number of individual substances from these groups that can be found in tobacco smoke and the main representatives of these groups in the particle phase (condensate) are shown in brackets.
- Compound classes
- Aliphatic (approx. 80 different substances, C 31 -C 33 - (n- and iso-) alkanes , C 5 -C 20 -alkenes),
- Aromates (approx. 100 substances, chrysene , benzo [ a ] pyrene , benzo [ c ] phenanthrene , benzo [ j ] fluoranthrene , - also heterocycles such as carbazole and many polycyclic aromatics (abbreviation: PAK, example: naphthalene) with a total of around 1, 25-2.85 micrograms / cigarette - measured in mainstream smoke),
- Alcohols (approx. 25 substances, ethanol , methanol , propanetriol (glycerine), ethanediol (ethylene glycol)),
- Aldehydes and ketones ( carbonyl derivatives , total approx. Substances, propanone (acetone), 2-butanone , 2-propenal (acrolein), propanal (propionaldehyde)),
- Carboxylic acids (55 substances, methane , ethane , propanoic acid ),
- Esters (approx. 270 substances, ethyl acetate , ethyl valerate , allyl caproate ),
- Phenols and phenol ethers (around 55 substances, hydroxymethylbenzenes ( cresols ), phenol , 2,4- and 2,5- xylenols ),
- Alkaloids and similar nitrogen bases (approx. 100 different substances, nicotine , nornicotine , anabasin , myosmin ),
- Heavy metal cations ( cadmium - 0.007-0.35 micrograms per cigarette -, mercury , copper , arsenic , nickel , zinc , lead , antimony and gold - the latter, however, only with 0.02 nanograms per cigarette),
- Radioisotopes (also 210 polonium , 210 lead , 226 radium and 228 radium and 40 potassium - and 228 thorium - annual mean exposure with 20 cigarettes daily approx. 0.287 mSv from 210 Po and 210 Pb),
- Peroxides , terpenes , sterols , nitrosamines and TSNA (N'-nitrosoanatabine (NAT), N'-nitrosonornicotine (NNN), 1-nitrosopyrrolidine), amino acids and proteins , pesticide residues and metabolic products of the same (“ pesticide metabolites”).
Fate and uptake of nicotine
30% to 35% of the nicotine contained in tobacco burns in the glowing zone, 40% goes into the sidestream smoke and 25% to 30% into the unfiltered mainstream smoke. Of this main stream portion, 30% - in absolute terms, 8% to 9% - remain in the tobacco stub in cigarettes without filters, 40% to 70% in filter cigarettes (absolute: 12% to 20%). In total, 14% to 20% of the nicotine from tobacco reaches the smoker's oral cavity (5% to 12% for filter cigarettes), of which up to 98% is absorbed when pulling the lungs, but only 5% when puffing in the mouth. The mainstream smoke of a filterless cigarette still contains 1.0 to 2.3 mg nicotine (as well as 10-23 mg carbon monoxide, also around 1 mg ethanal, 100 to 1000 micrograms acetic acid, 100 to 600 micrograms nitrogen oxide, 400 to 500 micrograms hydrocyanic acid ( Hydrogen cyanide), 20 to 50 micrograms of benzene, 60 to 100 micrograms each of acrolein and phenol and 70 to 100 micrograms of formaldehyde). In 1961 the smoke from a cigarette contained an average of 1.44 mg of nicotine and in 1990 it contained an average of 0.86 mg of nicotine.
Of the average of 860 micrograms of nicotine per cigarette, 43 micrograms to 103 micrograms enter the body directly through the oral cavity and when inhaling deeply “on the lungs”. 10 to 20 seconds after inhalation, the nicotine arrives in the brain. After about two hours, half of the amount of nicotine ingested is broken down by the body into so-called metabolites .
Regulation of tobacco smoke as an air pollutant
In 2006, the US state of California decided to include tobacco smoke in the list of "toxic air pollutants", which is also legally equated with other dangerous toxins.
ingredients
The mainstream smoke of a filterless cigarette contains between 15 milligrams and 40 milligrams of biologically active pollutants and toxins (“toxic substances”). With modern analytical methods, up to 9,600 different substances from different substance classes can be detected in tobacco smoke.
| substance |
salary |
substance |
salary |
|---|---|---|---|
| Carbon monoxide | < 10,000 | magnesium ionized | 0.07 |
| Nicotine | < 1,000 | antimony ionized | 0.052 |
| acetaldehyde | 500… 1,200 | Pyrene | 0.05 ... 1.01 |
| Hydrogen cyanide | 400 ... 500 | Benzo [ a ] fluorene | 0.04 ... 0.18 |
| Hydroquinone | 110 ... 300 | Iron ionizes | 0.042 |
| acetic acid | 100 ... 1,000 | o -Toluidine | 0.03 ... 0.16 |
| Nitrogen oxides | 100 ... 600 | Anthracene | 0.02 ... 0.23 |
| Catechol | 100 ... 360 | Benzo [ b ] fluorene | 0.02 |
| acetone | 100 ... 250 | Fluoranthene | 0.01 ... 0.27 |
| Methanol | 90 ... 180 | Hydrazine | 0.03 ... 0.04 |
| Formic acid | 80 ... 600 | Urethane | 0.02 ... 0.04 |
| formaldehyde | 70 ... 100 | Lead ionized | 0.017 ... 0.98 |
| potassium ionized | 70 | arsenic | 0.012 ... 0.022 |
| phenol | 60-140 | Dibenzo [ a , j ] anthracene | 0.01 ... 0.03 |
| Propenal | 60 ... 100 | cadmium | 0.007 ... 0.35 |
| ammonia | 50… 130 | 1-nitrosopyrrolidine | 0.006 ... 0.11 |
| 3- and 4-cresol | 40 ... 80 | Benzo [ a ] pyrene | 0.005 ... 0.078 |
| 3-methylpyridine | 20 ... 36 | Dibenzo [ a , h ] anthracene | 0.004 |
| Pyridine | 16 ... 40 | mercury | 0.004 |
| Nitrosamines (total) | 8.5 and less | Benzo [ a ] anthracene | 0.004-0.076 |
| N'-nitrosonornicotine | 3.7 and less | 1-methylchrysen | 0.003 |
| sodium ionized | 1.3 | Benzo [ ghi ] perylene | 0.003 ... 0.039 |
| Carbazole | 1.0 | Anthanthracene | 0.002 ... 0.022 |
| aluminum ionized | 0.22 | 4-aminobiphenyl | 0.002 ... 0.005 |
| N'-nitrosoanatabine | 0.2 ... 4.6 | Vinyl chloride | 0.001 ... 0.01 |
| 2-nitropropane | 0.2 ... 2.2 | N'-nitrosodiethylamine | 0.001 ... 0.02 |
| copper ionized | 0.19 | silver | 0.0012 |
| Zinc (ionic) | 0.12 ... 1.21 | gold | 0.00002 |
| Phenanthrene | 0.08 ... 0.62 | chrome | 0.004 |
The Terry Report evaluated the scientific findings of more than 7000 publications: Since 1964, it has been scientifically proven that cigarette smoking leads to a significantly increased incidence of lung tumors (lung cancer). Larynx, oral cavity, esophagus, bladder and pancreas tumors can also be caused by tobacco smoke.
Cancer risk
The main carcinogens ( carcinogens ) are the polycyclic aromatics (PAH) such as anthracene , benzo (a) fluorene, benzo (a) pyrene, phenanthrene, pyrene etc. as well as the tobacco-specific nitrosamines (TSNA, N-nitroso compounds) like that N-nitroso-dimethylamine, -methylethylamine, -nornicotine, -diethanolamine and 1-nitrosopyrrolidine and -piperidine. Their carcinogenic potential is supported by cocarcinogens, heavy metals, aromatic amines (such as aniline) and radioisotopes ( 210 polonium: 0.411 picocuria per gram of tobacco in tobacco smoke). Benzo (a) pyrene damages the gene p53, which is responsible for defense against cancer .
In addition, around 10 14 free oxygen radicals are generated per cigarette puff, which - like nitrosation processes inside the body ("endogenous") - can probably contribute to the development of cancer.
Since the sidestream smoke gets unfiltered into the ambient air at the workplace, a chapter "Passive smoking" was added to Section IIIB of the MAK list in 1985 (see also MAK values). In addition to the tumorigenic effects of tobacco smoke, active and passive smokers are more susceptible to heart attacks, heart disease and arteriosclerosis ("hardening of the arteries") as well as to throat, stomach and intestinal diseases (the latter mainly caused by nicotine and carbon monoxide) and bronchial diseases ("smoker's cough") ) detectable.
bronchitis
Bronchitis producing effect of tobacco smoke is attributed to phenol and acid components in the tobacco smoke and the carbonyl compounds ( alkanals and alkanones ), in addition to suppress hydrocyanic acid and acrolein regeneration and self-cleaning of the cilia ( epithelium ) in the respiratory tract, as well as the formation of the white blood cells ( leukocytes ). Thiocyanates are also increasingly detectable in the saliva of smokers . Compared to non-smokers, the organisms of smokers have a lower body weight and an increased basal metabolic rate, which suggests increased enzyme activity.
Substitute solutions
To reduce the pollutant content in the smoke, filters made of cellulose acetate were developed, which hold back part of the nicotine and the particle phase ("tar", condensate). These hold back around 40% to 70% of the particles and up to 80% of the phenols in tobacco smoke. Additional activated carbon filters hold back up to 85% of the gas phase components.
In addition, in the last few years of the 20th century, intensive experiments were carried out with semi-synthetic tobacco: 20-25% synthetic substances (partially oxidized polysaccharides or tobacco substitutes such as NSM, RCN and Cytrel) and artificial flavorings are added to the tobacco - but these have extremely low-nicotine products No acceptance found among consumers, so that average values of 0.6–0.8 mg nicotine and 12–14 mg condensate per cigarette have remained. Likewise, cigarettes made from tobacco, which has been made nicotine-free through genetic manipulation, have remained a niche product.
Medical consequences of tobacco smoke inhalation
With a daily consumption of 20 cigarettes over 20 years, a total of six kilograms of smoke dust is absorbed through the lungs, as well as around one cup of condensate annually. This type of smoke inhalation shortens the lifespan - statistically speaking - by six years (for 10 cigarettes a day the measured value is 3 years, for 40 cigarettes / day around eight years). Carbon monoxide causes a lack of oxygen in all organs - and even substances such as hydrogen cyanide, benzene and benzopyrene can be detected in cigarette smoke.
Inhaling tobacco smoke is an established risk factor for various types of cancer, lung diseases and diseases of the cardiovascular system, among other things. In cancer development and adhering to the tobacco plays 210 polonium a role. In addition, the activity of more than 300 genes and entire gene networks is negatively influenced by tobacco consumption.
See also
- Ordinance on tobacco products Which auxiliaries and additives may be used in tobacco?
- Tobacco addiction
Web links
- Allum (allergy, environment and health): information on tobacco smoke
- Database of additives in tobacco (Federal Ministry of Food and Agriculture)
- Qualitative and quantitative information on toxins in tobacco smoke
Individual evidence
- ↑ a b Alan Rodgman; Thomas A. Perfetti: The Chemical Components of Tobacco and Tobacco Smoke , second Edition (February 25, 2013).
- ↑ Section Production, Composition, Use and Regulations (PDF; 1.4 MB) of IARC Monograph 83 from 2004.
- ↑ a b c J. Falbe, Manfred Regitz (ed.): Römpp Lexikon Chemie . Thieme, Stuttgart / New York, 9th edition, pp. 4434-4438, ISBN 3-440-04516-1 .
- ↑ a b B. Mayer: How much nicotine kills a human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century. In: Archives of Toxicology . Volume 88, Number 1, January 2014, pp. 5-7, doi: 10.1007 / s00204-013-1127-0 . PMID 24091634 . PMC 3880486 (free full text).
- ↑ Paul Obrecht: Clinical Cancerologie. In: Ludwig Heilmeyer (ed.): Textbook of internal medicine. Springer-Verlag, Berlin / Göttingen / Heidelberg 1955; 2nd edition, ibid. 1961, pp. 352-375, here: p. 364.
- ↑ a b c d e Technical rules for hazardous substances 900: Occupational exposure limits. (PDF; 631 kB) BAuA , November 4, 2016, accessed on January 27, 2017 .
- ↑ A. Savidou, K. Kehagia, K. Eleftheriadis: Concentration levels of 210Pb and 210Po in dry tobacco leaves in Greece. In: Journal of Environmental Radioactivity. 85, 2006, pp. 94-102, doi: 10.1016 / j.jenvrad.2005.06.004 .
- ↑ S. Feng, S. Kapur, M. Sarkar, R. Muhammad, P. Mendes, K. Newland, HJ Roethig: Respiratory retention of nicotine and urinary excretion of nicotine and its five major metabolites in adult male smokers. In: Toxicology letters. Volume 173, number 2, September 2007, pp. 101-106, doi: 10.1016 / j.toxlet.2007.06.016 . PMID 17716838 .
- ^ Serban C. Moldoveanu and F. Kelley St. Charles: Differences in the Chemical Composition of the Particulate Phase of Inhaled and Exhaled Cigarette Mainstream Smoke. In: Contributions to tobacco research. Volume 22, Issue 4,2007, pp. 290-302 (PDF; 901 kB).
- ↑ J. Le Houezec: Role of nicotine pharmacokinetics in nicotine addiction and nicotine replacement therapy: a review. In: The international journal of tuberculosis and lung disease: the official journal of the International Union against Tuberculosis and Lung Disease. Volume 7, Number 9, September 2003, pp. 811-819, PMID 12971663 . (Review).
- ↑ Tobacco consumption disrupts the activity of important genes , spiegel.de. July 15, 2010. Retrieved July 24, 2010.