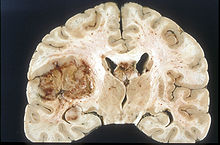
Disruption of the blood-brain barrier
Disorders of the blood-brain barrier can be caused by a number of different diseases. The blood-brain barrier itself can also be the starting point for some very rare neurological diseases. The blood-brain barrier is an in brain existing physiological barrier between the bloodstream and the central nervous system (CNS), which serves the environmental conditions ( homeostasis maintain) in the brain and of which delineate the blood.
Diseases directly associated with the blood-brain barrier
While a large number of neurological diseases disrupt or damage the blood-brain barrier, the blood-brain barrier itself is only the starting point of a disease in some extremely rare, genetically determined syndromes.
GLUT1 deficit syndrome
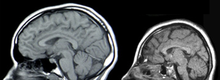
The GLUT1 deficiency syndrome is an extremely rare, autosomal - dominant inherited disease. The disease manifests itself neonatally or in infancy and is caused by a lack of GLUT1 transporters in the endothelium of the blood-brain barrier. The deficit in GLUT1 transporters is mostly caused by new mutations in the SLC2A1 gene. As a result, the brain is not adequately supplied with D- glucose and the affected patients show, among other things, microcephaly , psychomotor retardation, ataxia and a number of other neurological disorders.
Biotin-responsive basal ganglia disease
As in the GLUT1 deficit syndrome, a mutation in a gene that codes for a transporter protein is the cause of the disease in biotin-responsive basal ganglia disease. This extremely rare disease - so far only a little more than ten patients with this disease are known - is caused by a defect in the SLC19A3 gene on chromosome 2, gene locus q36.3, which codes for a folate transporter. As a result of this genetic defect, the brain in the affected patients is not adequately supplied with biotin, which manifests itself in a subacute encephalopathy and a variety of neurological symptoms. The disease can be treated well through supplementation with biotin.
Hereditary folate malabsorption
The autosomal recessive inherited folate malabsorption (Latin malabsorption = "poor absorption") is an extremely rare disease. With her the brain is undersupplied with folic acid. The cause here is a defect in the PCFT gene (SLC46A1), which codes for a proton-dependent folate transporter.
MDR1 defect in Collies and dog breeds derived from it
Some dog breeds , all of which are derived from the Collie , are hypersensitive to some drugs. The cause of this hypersensitivity is a defect in the mdr1 gene, which codes for P-glycoprotein. Due to this genetic defect - called mdr1-1Delta mutation - the efflux at the blood-brain barrier is largely prevented and the corresponding drugs can reach the central nervous system by diffusion. The mdr1-1Delta mutation probably originated in a single breeding dog in the middle of the 19th century and was passed on from this to many generations. Particularly when antiparasitics , cytostatics and antibiotics are administered, the affected dogs have severe neurotoxic side effects.
Diseases indirectly associated with the blood-brain barrier
The disruption of the protective effect of the blood-brain barrier is a complication of many neurodegenerative diseases and brain injuries. Some peripheral diseases, such as diabetes mellitus or inflammation , have a detrimental effect on the functioning of the blood-brain barrier.
Other diseases, on the other hand, disrupt the function of the endothelia from "inside out", that is, the integrity of the blood-brain barrier is impaired by influences that come from the extracellular matrix. An example of this is glioblastoma.
In contrast, a number of diseases in the brain manifest themselves in that certain pathogens can cross the blood-brain barrier. These include, for example, the HI virus, the human T-lymphotropic virus 1 , the West Nile virus and bacteria such as Borrelia burgdorferi , Neisseria meningitidis or Vibrio cholerae .
In the case of multiple sclerosis, the "pathogens" are cells of the body's own immune system that cross the blood-brain barrier. Likewise, in some non-cerebral tumors, metastatic cells cross the blood-brain barrier and can lead to metastases in the brain.
Diabetes mellitus
Diabetes mellitus is a metabolic disease that results in a number of functional and structural changes in various organs and the central nervous system. Significant changes also take place at the blood-brain barrier, which impair both the barrier effect and the transport functions. The physicochemical properties of the plasma membrane and the tight junctions of the endothelia are changed.
With increasing duration of the diabetes, the barrier effect of the endothelia diminishes measurably. This was initially determined in an animal model using various marker molecules of different sizes. This mainly affects the structural proteins of the tight junctions. The changes in tight junctions are not evenly distributed across the brain. The capillaries of the midbrain are particularly affected , while other areas show no changes. The effects of these structural changes - induced by diabetes mellitus - are still largely unexplored. The effects of diabetes on peripheral endothelia are well known, where their dysfunction leads to the secondary effects associated with diabetes, such as blindness , chronic kidney failure and neuropathy . Current clinical studies show that diabetics have an increased risk of vascular dementia , ventricular hypertrophy , lacunar infarct and cerebral haemorrhage , the causes of which are seen in changes in the blood-brain barrier. Obviously, type 2 diabetics are also predisposed to the development of Alzheimer's disease .
multiple sclerosis
Multiple sclerosis (MS) is an autoimmune inflammatory demyelinating disease of the central nervous system (CNS) in which lymphocytes and macrophages infiltrate the CNS. During the infiltration, the body's own defense cells cross the blood-brain barrier. The T lymphocytes then attack the myelin sheaths located in the interstitium of the brain or spinal cord .
The infiltration of the lymphocytes takes place via the endothelial cells of the blood-brain barrier. The exact mechanism of this process is not yet fully understood. The inflammatory reaction of the tissue releases cytokines which stimulate the endothelial cells to express the adhesion molecules ICAM1 and VCAM1. The cytokines also cause the endothelia to synthesize chemokines and chemoattractors , which are presented on the luminal side of the capillaries. Chemoattractors present on the abluminal side are transported to the luminal side. The leukocytes rolling along the capillary walls then bind to the endothelia and special adhesion molecules are expressed to anchor the leukocytes to the endothelium. This is followed by either paracellular or transcellular transmigration . Both ways are possible and both ways could be proven.
In paracellular transmigration - called leukodiapedesis - the leukocyte opens the tight junctions by releasing signal and adhesion molecules. The endothelial cells contract, the tight junctions open and the leukocyte can penetrate the interstitium of the central nervous system. The process itself is highly complex and only reproduced here in a very simplified manner. The transcellular route is also possible. Several vesicles and vacuoles in the endothelium form transendothelial cell channels. These vesiculovacuolar organelles (VVO) are found in normal blood vessels as well as in tumor cells. They enable the active transport of fluids, but also cells, across the blood-brain barrier. The transport is mediated by the Vascular Endothelial Growth Factor (VEGF). VEGF is a vasoactive, permeability-promoting protein. The transcellular transport via the vesiculovacuolar organelles is obviously also a mechanism with which pathogens (bacteria and viruses) can cross the blood-brain barrier.
In general, the malfunction of the blood-brain barrier is one of the characteristics of multiple sclerosis. The breakdown of the blood-brain barrier is mainly young patients with a particularly aggressive form of relapsing remitting multiple sclerosis (RR-MS, English. Relapsing-remitting multiple sclerosis ) affected. The phenomenon can be made visible during magnetic resonance imaging by diffusing in a paramagnetic contrast agent, for example gadopentetate dimeglumine (Gd-DTPA). In terms of sensitivity, the method is superior to the monitoring of marker molecules such as cytokines or chemokines.
The cause of the malfunction of the blood-brain barrier in MS is a decreased expression of the tight junction proteins of the endothelial cells. The changes at the tight junctions can be observed not only in the active lesions , but also - albeit less frequently - in the inactive lesions and even in the white matter . The changes in tight junctions worsen homeostasis and apparently have an impact on disease progression, repair mechanisms, and drug delivery.
Ischemic Stroke
In an ischemic stroke (cerebral infarction), changes in the endothelia of the blood-brain barrier occur as a result of cerebral ischemia (local blood loss in the brain) and the subsequent reperfusion (restoration of the blood supply, see also reperfusion damage). These changes take place in two phases. The first phase occurs a few minutes after reperfusion, the second phase several hours after ischemia. The release of oxidants, proteolytic enzymes and cytokines massively changes the permeability of the blood-brain barrier and leads to the formation of edema in the brain. As a result of the edema, activated leukocytes release matrix metalloproteases (MMP), which in turn break down the basal lamina and protein complexes of the tight junctions. MMP9 (gelatinase B) in particular plays an important role here. As a result of the opening of the blood-brain barrier, the neurotoxic tissue-specific plasminogen activator (t-PA) can penetrate the brain by means of passive diffusion.
Obviously, leukocytes that migrate into the parenchyma of the brain also contribute to the damage caused by ischemia and reperfusion. The leukocytes overcome the endothelium - as in multiple sclerosis - through transendothelial migration.
Bacterial infections of the central nervous system (meningitis)
Only a few pathogens transmitted through the blood are in principle able to cross the blood-brain barrier. These include meningococci ( Neisseria meningitidis ), streptococci ( Streptococcus agalactiae ), pneumococci ( Streptococcus pneumoniae ), Haemophilus ( Haemophilus influenzae ), Listeria ( Listeria monocytogenes ) and coli bacteria ( Escherichia coli-K1 ), all of which cross the barrier Can cause meningitis ( meningitis ). The exact mechanisms by which these pathogens cross the blood-brain barrier are not yet fully understood. The pathogens use different ways to overcome the endothelia. Inflammatory processes play an essential role in this.
First, the pathogens, such as L. monocytogenes , attach themselves to the endothelia and then release a number of lipopolysaccharides and toxins, which in turn leads to the production of various cytokines (TNF-α, IL1β, PAF, TGFβ1), matrix -Metalloproteases and caspases stimulates. The release of these proteins leads to a significant increase in the permeability of the capillaries. The increased permeability of the endothelium enables the transendothelial migration of leukocytes into the brain. These in turn release cytokines and matrix metalloproteases, which further activate the endothelia and considerably intensify the inflammatory reaction in the affected areas of the central nervous system.
S. pneumoniae , on the other hand, is able to produce transmembrane pores in the endothelium through the secretion of pneumolysin , an enzyme from the group of hemolysins . Via released endotoxins , S. pneumoniae can even trigger apoptosis (programmed cell death) in the endothelia . Also S. agalactiae. directly attacks the endothelia of the blood-brain barrier.
N. meningitidis , S. pneumoniae and E. coli K1 are also able to cross the blood-brain barrier with the help of transendothelial migration like leukocytes. After their attachment via type IV pili to the endothelial cells of the blood-brain barrier, they trigger a signal cascade similar to leukocytes, which ultimately enables these pathogens to migrate into the central nervous system.
Viruses and the blood-brain barrier
Viruses are also among the pathogens that are able to cross the blood-brain barrier. As with bacteria, several mechanisms are known for their passage into the brain.
Neurotropic viruses such as the cytomegalovirus , the human immunodeficiency virus (HIV) and the human T-lymphotropic virus 1 (HTLV-1) can cross the blood-brain barrier as free viruses by means of macropinocytosis.
In addition, the transendothelial migration of infected leukocytes plays an essential role in the passage of the blood-brain barrier. This mechanism has been described in the case of the cytomegalovirus and the HI virus, among others. Cells infected with HIV or HTLV-I produce large amounts of matrix metalloproteases, which in turn attack the basal lamina and thus enable the infected cells to migrate into the brain.
HIV
The HI virus crosses the blood-brain barrier shortly after infection. Obviously, several independent mechanisms play a role. Migration via infected monocytes and T lymphocytes (“ Trojan horses ”) is of crucial importance in the case of the HI virus for the infection of the central nervous system in the late stages of AIDS . The mechanism by which the virus with the help of systemic leukocytes crosses the blood-brain barrier and causes lasting damage is still unknown. The TAT protein, a gene product of the HI virus, and gp120 , a glycoprotein encoded by HIV, play an important role in the subsequent damage to the central nervous system. As a toxin, TAT is able to directly and indirectly produce oxidative stress and inflammatory reactions in the endothelium. Oxidative stress in particular is an important factor in the development of HIV-related dementia . In addition, TAT and gp120 are able to trigger apoptosis in the endothelial cells of the blood-brain barrier.
The opening of the blood-brain barrier not only contributes to the progression of the infection with HI viruses in the brain, but also leads to complications in the course of the disease and to problems with antiretroviral therapy .
Human T-lymphotropic virus 1
The HTL virus 1 is also able to cross the blood-brain barrier. By secreting interleukin-1α and TNF-α, the virus increases both the paracellular permeability of the endothelia and the ability to migrate transcellularly. The gene expression of tight junction proteins, such as ZO-1, is also influenced by this. Obviously, the lymphocytes infected with HTLV-1 are responsible for the opening of the blood-brain barrier and the associated further infiltration of lymphocytes, but also of neurotoxic plasma proteins, which ultimately lead to HTLV-1-associated myelopathy .
West Nile Virus
The West Nile virus is an enveloped RNA virus that is also able to cross the blood-brain barrier and cause encephalitis or meningitis . The virus infects macrophages or dendritic cells in the peripheral lymphatic tissue . These then emit TLR3- dependent ( toll-like receptor ) antiviral and immunomodulating cytokines such as interferons , interleukin-6 and TNF-α , which prevents further infection in the peripheral tissue. The TLR3-dependent release of TNF-α enables the West Nile virus to cross the blood-brain barrier. Changes in the tight junctions caused by TNF-α play an important role here. Through these changes, either the free viruses or virus-infected leukocytes can penetrate the central nervous system.
Neurodegenerative Diseases
Up until the beginning of the 21st century, it was assumed that neurodegenerative diseases such as Alzheimer's and Parkinson's disease had no effect on the blood-brain barrier. More recent research results refute this thesis. With the help of contrast-enhanced magnetic resonance tomography and biochemical examinations of the CSF , for example, functional changes in the blood-brain barrier in the direction of increased permeability could be determined in Alzheimer's patients compared to a group of non-sick people of the same age. The increased permeability is apparently already present at a very early stage of the disease. The consequences of these findings are currently still being discussed controversially. The changes in the blood-brain barrier could influence the progression of the disease as well as possible future therapeutic approaches.
Glioblastoma and other primary brain tumors
The growth of a tumor is accompanied by the formation of new blood vessels ( neovascularization ) at a very early stage so that the tumor can be supplied with sufficient oxygen and nutrients. Neovascularization begins at a diameter of 1 to 2 mm. The newly formed blood vessels show considerable structural differences compared to normal blood vessels. In brain tumors , these structural differences lead to significant local changes in the blood-brain barrier. Especially in glioblastoma , the neovascularization is very pronounced and one of the factors in the aggressive growth in this cancer.
The newly formed blood vessels of primary brain tumors have a more tortuous structure than the normal blood vessels of the brain. The endothelia are covered by a deformed basal lamina and no longer express the tight junction proteins claudin-3 and occludin. In contrast, the vascular endothelial growth factor (VEGF) is produced in the tumors in large quantities , which promotes the endocytosis of the cell adhesion protein VE-cadherin and thereby further increases the permeability of the endothelial cells. The degree of expression of occludin correlates inversely proportionally with the grading and permeability of the affected endothelia for contrast media, which cannot cross a healthy blood-brain barrier. The increased permeability of the endothelial cells for certain contrast media is used in diagnostics (see the section on human diagnostics ).
In therapy, the blood vessels of the brain tumors are a potential target for angiogenesis inhibitors . The target structures are, inter alia, the α v β 3 - integrin ( Cilengitide ) and VEGF ( bevacizumab ).
Metastatic tumor cells
Metastatic tumor cells, especially those of bronchial carcinomas , breast carcinomas and malignant melanomas , are able to cross the blood-brain barrier and form cerebral metastases with an invariably poor prognosis . Brain metastases form in around 20 to 40% of all cancer patients . The vascular endothelial growth factor also plays a role in the migration of these cells into the central nervous system . (VEGF) plays an important role. VEGF is particularly strongly expressed by breast cancer cells and melanoma cells.
Some metastatic tumor cells - similar to activated leukocytes - express a whole range of adhesion molecules on their cell surface. Together with chemokine receptors , such as CXCR4 , which binds to the chemokine CXCL12 , which is strongly expressed in the brain , this gives the metastatic tumor cells the opportunity to attach themselves to the endothelium of the capillary vessels. After the attachment, the enothelia are activated by the tumor cells, which enables migration into the central nervous system. In vitro , the function of CXCR4 could be demonstrated by blocking the intracellular pathway using phosphoinositide-3-kinases . Tumor cells of the breast cancer cell line DU4475 with blocked CXCR4 could no longer migrate through cultured endothelia.
Specialist literature
- D. Kobiler et al. a .: Blood-brain Barrier. Verlag Springer, 2001, ISBN 0-306-46708-9 .
- WM Pardridge: Introduction to the Blood-brain Barrier. Cambridge University Press, 1998, ISBN 0-521-58124-9 .
Web links
Individual evidence
- ↑ J. Klepper et al. a .: Autosomal dominant transmission of GLUT1 deficiency. In: Hum Molec Genet. 10, 2001, pp. 63-68, PMID 11136715 .
- ↑ DC De Vivo u. a .: Defective glucose transport across the blood-brain barrier as a cause of persistent hypoglycorrhachia, seizures, and developmental delay. In: NEJM . 325, 1991, pp. 703-709, PMID 1714544 .
- ↑ orpha.net: Encephalopathy due to GLUT1 defect. Accessed March 3, 2009.
- ↑ Glucose Transport Defect, Blood-Brain Barrier. In: Online Mendelian Inheritance in Man . (English), accessed March 3, 2009.
- ↑ WQ Zeng u. a .: Biotin-responsive basal ganglia disease maps to 2q36.3 and is due to mutations in SLC19A3. In: Am J Hum Genet. 77, 2005, pp. 16-26, PMID 15871139 .
- ↑ PT Ozand et al. a .: Biotin-responsive basal ganglia disease: a novel entity. In: Brain. 121, 1998, pp. 1267-1279, PMID 9679779 .
- ↑ Basal Ganglia Disease, biotin-responsive. In: Online Mendelian Inheritance in Man . (English), accessed March 3, 2009.
- ↑ a b R. Zhao u. a .: The spectrum of mutations in the PCFT gene, coding for an intestinal folate transporter, that are the basis for hereditary folate malabsorption. In: Blood . 110, 2007, pp. 1147-1152, PMID 17446347 .
- ^ PC Su: Congenital folate deficiency. In: NEJM. 294, 1976, p. 1128, PMID 176588 .
- ↑ Folic Acid, transport Defect Involving. In: Online Mendelian Inheritance in Man . (English), accessed March 3, 2009.
- ↑ S. Fecht and O. Distl: Review of prevalence, genetic aspects and adverse effects of the mdr1-1 Delta mutation in dogs. In: Dtsch Tierarztl Wochenschr. 115, 2008, pp. 212-219, PMID18605373 (review).
- ↑ J. Geyer et al. a .: MDR1 defect. Multiple drug hypersensitivity in British Sheepdogs. In: Small animal specifically. 9, 2006, pp. 16-20.
- ↑ BT Hawkins and RD Egleton: Pathophysiology of the blood-brain barrier: animal models and methods. In: Curr Top Dev Biol. 80, 2008, pp. 277-309, PMID 17950377 (review).
- ↑ a b c d e f g h i j k l m n o N. Weiss, F. Miller, S. Cazaubon, PO Couraud: The blood-brain barrier in brain homeostasis and neurological diseases. In: Biochim. Biophys. Acta 1788, 2009, pp. 842-857, PMID 19061857 (review).
- ↑ MH Horani and AD Mooradian: Effect of diabetes on the blood brain barrier. In: Curr Pharm Des. 9, 2003, pp. 833-840, PMID 12678883 (review).
- ↑ a b J. D. Huber u. a .: Streptozotocin-induced diabetes progressively increases blood-brain barrier permeability in specific brain regions in rats. In: Am J Physiol Heart Circ Physiol. 291, 2006, pp. H2660-H2668, PMID 16951046 .
- ↑ JM Chehade et al. a .: Diabetes-related changes in rat cerebral occlusion and zonula occludens-1 (ZO-1) expression. In: Neurochem Res. 27, 2002, pp. 249-252, PMID 11958524 .
- ^ WA Banks: The dam breaks: disruption of the blood-brain barrier in diabetes mellitus. ( Memento of the original from January 9, 2009 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. In: Am J Physiol Heart Circ Physiol. 291, 2006, pp. 2595-2596, PMID 16877556 (review).
- ^ JD Huber: Diabetes, cognitive function, and the blood-brain barrier. In: Curr Pharm Des. 14, 2008, pp. 1594-1600, PMID 18673200 (review).
- ^ PA Whitmer: Type 2 diabetes and risk of cognitive impairment and dementia. In: Curr Neurol Neurosci Rep. 7, 2007, pp. 373-380, PMID 17764626 (review).
- ^ J. Correale and A. Villa: The blood-brain-barrier in multiple sclerosis: functional roles and therapeutic targeting. In: Autoimmunity. 40, 2007, pp. 148-160, PMID 17453713 .
- ↑ JJ Campbell et al. a .: Biology of chemokine and classical chemoattractant receptors. Differential requirements for adhesion-triggering versus chemotactic responses in lymphoid cells. In: J Cell Biol 134, 1996, pp. 255-266, PMID 8698820 .
- ↑ JJ Campbell et al. a .: Chemokines and the arrest of lymphocytes rolling under flow conditions. In: Science 279, 1998, pp. 381-384, PMID 9430588 .
- ↑ a b c d K. Ley u. a .: Getting to the site of inflammation: the leukocyte adhesion cascade updated. In: Nature Reviews Immunology 7, 2007, pp. 678-689, PMID 17717539 (review).
- ^ CV Carman and TA Springer: Trans-cellular migration: cell-cell contacts get intimate. In: Curr Opin Cell Biol. 20, 2008, pp. 533-540, PMID 18595683 (review).
- ↑ AM Dvorak u. a .: The vesiculo-vacuolar organelle (VVO): A distinct endothelial cell structure that provides a transcellular pathway for macromolecular extravasation. ( Memento of the original from May 25, 2008 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. In: J Leukoc Biol. 59, 1996, pp. 100-115, PMID 8558058 .
- ↑ AS Lossinsky and RR Shivers: Structural pathways for macromolecular and cellular transport across the blood-brain barrier during inflammatory conditions. In: Histol Histopathol. 19, 2004, pp. 535-564, PMID 15024715 (review).
- ^ S. Nag: The blood-brain barrier. Humana Press, 2003, ISBN 1-58829-073-5 , p. 76.
- ↑ LA Stone: Blood-brain barrier disruption on contrast-enhanced MRI in patients with mild relapsing-remitting multiple sclerosis: relationship to course, gender and age. In: Neurology 45, 1995, pp. 1122-1126, PMID 7783875 .
- ↑ E. Waubant: Biomarkers indicative of blood-brain barrier disruption in multiple sclerosis. In: Dis Markers. 22, 2006, pp. 235-244, PMID 17124345 (review).
- ^ S. McQuaid et al. a .: The effects of blood-brain barrier disruption on glial cell function in multiple sclerosis. In: Biochem Soc Trans 37, 2009, pp. 329-331, PMID 19143657 (review).
- ↑ L. Belayev et al. a .: Quantitative evaluation of blood-brain barrier permeability following middle cerebral artery occlusion in rats. In: Brain Res. 739, 1996, pp. 88-96, PMID 8955928 .
- ↑ J. Aronowski et al. a .: Reperfusion injury: demonstration of brain damage produced by reperfusion after transient focal ischemia in rats. In: J Cereb Blood Flow Metab 17, 1997, pp. 1048-1056, PMID 9346429 .
- ↑ U. Dirnagl u. a .: Pathobiology of ischaemic stroke: an integrated view. In: Trends Neurosci. 22, 1999, pp. 391-397, PMID 10441299 (review).
- ^ VW Yong: Metalloproteinases: Mediators of Pathology and Regeneration in the CNS. In: Nat Rev Neurosci . 6, 2005, pp. 931-944, PMID 16288297 (review).
- ↑ M. Asahi et al. a .: Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. In: J Neurosci. 21, 2001, pp. 7724-7732, PMID 11567062 .
- ↑ K. Benchenane et al. a .: Tissue-type plasminogen activator crosses the intact blood-brain barrier by low-density lipoprotein receptor-related protein-mediated transcytosis. In: Circulation 111, 2005, pp. 2241-2249, PMID 15851587 .
- ↑ S. Kuroda and BK Siesjö: reperfusion damage Following focal ischemia: pathophysiology and therapeutic windows. In: Clin Neurosci. 4, 1997, pp. 199-212, PMID 9186042 (review).
- ↑ AM Planas u. a .: Signaling pathways mediating inflammatory responses in brain ischaemia. In: Biochem Soc Trans 34, 2006, pp. 1267-1270, PMID 17073799 (review).
- ↑ PJ Lindsberg u. a .: Endothelial ICAM-1 expression associated with inflammatory cell response in human ischemic stroke. In: Circulation. 94, 1996, pp. 939-945, PMID 8790029 .
- ↑ RL Zhang u. a .: The temporal profiles of ICAM-1 protein and mRNA expression after transient MCA occlusion in the rat. In: Brain Res. 682, 1995, pp. 182-188, PMID 7552309 .
- ↑ SL Leib u. a .: Matrix metalloproteinases contribute to brain damage in experimental pneumococcal meningitis, Infect. In: Immun. 68, 2000, pp. 615-620, PMID 10639424 .
- ↑ G. Zysk et al. a .: Pneumolysin is the main inducer of cytotoxicity to brain microvascular endothelial cells caused by Streptococcus pneumoniae. In: Infect Immun. 69, 2001, pp. 845-852, PMID 11159977 .
- ↑ A. Halle: Streptococcus pneumoniae induces apoptosis in cerebral endothelial cells: the role of bacterial toxins. Dissertation, Medical Faculty of the Charité, 2005.
- ↑ DD Bannerman and SE Goldblum: Direct effects of endotoxin on the endothelium: barrier function and injury. In: Lab Invest. 79, 1999, pp. 1181-1199, PMID 10532583 (review).
- ↑ KS Doran et al. a .: Blood-brain barrier invasion by group B streptococcus depends upon proper cell-surface anchoring of lipoteichoic acid. In: J Clin Invest. 115, 2005, pp. 2499-2507, PMID 16138192 .
- ↑ HC Maisey u. a .: Group B streptococcal pilus proteins contribute to adherence to and invasion of brain microvascular endothelial cells. In: J Bacteriol. 189, 2007, pp. 1464-1467, PMID 17041051 .
- ↑ KS Kim: Pathogenesis of bacterial meningitis: from bacteremia to neuronal injury. In: Nat Rev Neurosci. 4, 2003, pp. 376-385, PMID 12728265 (review).
- ^ X. Nassif et al. a .: How do extracellular pathogens cross the blood-brain barrier? In: Trends Microbiol. 10, 2002, pp. 227-232, PMID 11973156 (review).
- ↑ a b D. T. Wu u. a .: Mechanisms of leukocyte trafficking into the CNS. In: J Neurovirol. 6, 2000, pp. 82-85, PMID 10871769 .
- ↑ GL Bentz u. a .: Human cytomegalovirus (HCMV) infection of endothelial cells promotes naive monocyte extravasation and transfer of productive virus to enhance hematogenous dissemination of HCMV. In: J Virol. 80, 2006, pp. 11539-11555, PMID 16987970 .
- ↑ WA Banks et al. a .: Transport of human immunodeficiency virus type 1 pseudoviruses across the blood-brain barrier: role of envelope proteins and adsorptive endocytosis. In: J Virol. 75, 2001, pp. 4681-4691, PMID 11312339 .
- ↑ AT Haase: Pathogenesis of lentivirus infections. In: Nature. 322, 1986, pp. 130-136, PMID 2425264 (review).
- ↑ R. Peluso et al. a .: A Trojan Horse mechanism for the spread of visna virus in monocytes. In: Virology. 147, 1985, pp. 231-236.
- ↑ JD Reuter et al. a .: CD4 + T-cell reconstitution reduces cytomegalovirus in the immunocompromised brain. In: J Virol. 79, 2005, pp. 9527-9539, PMID 16014915 .
- ↑ A. Alexaki and B. Wigdahl: HIV-1 infection of bone marrow hematopoietic progenitor cells and their role in trafficking and viral dissemination. In: PLoS Pathog 4, 2008, p. E1000215, PMID 19112504 .
- ↑ K. Conant et al. a .: Cerebrospinal fluid levels of MMP-2, 7, and 9 are elevated in association with human immunodeficiency virus dementia. In: Ann Neurol. 46, 1999, pp. 391-398, PMID 10482270 .
- ↑ a b J. R. Berger and M. Avison: The blood brain barrier in HIV infection. In: Front Biosci. 9, 2004, pp. 2680-2685, PMID 15358591 .
- ↑ HS Nottet u. a .: Mechanisms for the transendothelial migration of HIV-1-infected monocytes into brain. In: J Immunol. 156, 1996, pp. 1284-1295, PMID 8558009 .
- ↑ a b T. A. Kim u. a .: HIV-1 Tat-mediated apoptosis in human brain microvascular endothelial cells. In: J Immunol. 170, 2003, pp. 2629-2637, PMID 12594291 .
- ↑ H. Wang et al. a .: Human immunodeficiency virus type 1 infection increases the in vivo capacity of peripheral monocytes to cross the blood-brain barrier into the brain and the in vivo sensitivity of the blood-brain barrier to disruption by lipopolysaccharide. In: J Virol. 82, 2008, pp. 7591-7600, PMID 18508884 .
- ↑ CB Pocernich u. a .: HIV-dementia, Tat-induced oxidative stress, and antioxidant therapeutic considerations. In: Brain Res Brain Res Rev. 50, 2005, pp. 14-26, PMID 15890409 (review).
- ↑ M. Toborek et al. a .: HIV-Tat protein induces oxidative and inflammatory pathways in brain endothelium. In: J Neurochem. 84, 2003, pp. 169-179, PMID 12485413 .
- ↑ FM Hofman et al. a .: Exogenous Tat protein activates human endothelial cells. Blood 92, 1993, pp. 2774-2780, PMID 7693046 .
- ↑ J. Steiner et al. a .: Oxidative stress and therapeutic approaches in HIV dementia. In: Antioxidant Redox Signal. 8, 2006, pp. 2089-2100, PMID 17034352 (review).
- ^ PV Afonso et al. a .: Alteration of blood-brain barrier integrity by retroviral infection. In: PLoS Pathog. 4, 2008, e1000205, PMID 19008946 .
- ^ PV Afonso et al. a .: Human blood-brain barrier disruption by retroviral-infected lymphocytes: role of myosin light chain kinase in endothelial tight-junction disorganization. In: J Immunol. 179, 2007, pp. 2576-2583, PMID 17675520 .
- ↑ P. Shapshak et al. a .: HTLV-III Can Cross the Blood-Brain Barrier. In: Annals of the New York Academy of Sciences. 529, 2006, pp. 291-294, doi: 10.1111 / j.1749-6632.1988.tb51485.x
- ↑ MS Diamond and RS Klein: West Nile virus: crossing the blood-brain barrier. In: Nature Medicine 10, 2004, pp. 1294-1295, PMID 15580248 .
- ^ R. Paterson: How West Nile virus crosses the blood-brain barrier. In: The Lancet Neurology 4, 2005, p. 18, PMID 15645594 .
- ↑ AJ Farrall and JM Wardlaw: Blood-brain barrier: Aging and microvascular disease - systematic review and meta-analysis. In: Neurobiol Aging. 30, 2009, pp. 337-352, PMID 17869382 .
- ↑ JM Starr u. a .: Blood-brain barrier permeability in Alzheimer's disease: a case-control MRI study. In: Psychiatry Res. 171, 2009, pp. 232-241, PMID 19211227 .
- ↑ BV Zlokovic: Neurovascular mechanisms of Alzheimer's neurodegeneration. In: Trends Neurosci. 28, 2005, pp. 202-208, PMID 15808355 (review).
- ↑ BS Desai et al. a .: Blood-brain barrier pathology in Alzheimer's and Parkinson's disease: implications for drug therapy. In: Cell Transplant. 16, 2007, pp. 285-299, PMID 17503739 .
- ↑ D. Hanahan and J. Folkman: Patterns and emering mechanisms of the angiogenic switch during tumorigenesis. In: Cell 86, 1996, pp. 353-364, PMID 8756718 (review).
- ↑ a b J. C. Anderson et al. a .: New molecular targets in angiogenic vessels of glioblastoma tumors. In: Expert Rev Mol Med. 10, 2008, p. E23, PMID 18684337 (review).
- ↑ H. Wolburg et al. a .: Localization of claudin-3 in tight junctions of the blood-brain barrier is selectively lost during experimental autoimmune encephalomyelitis and human glioblastoma multiforme. In: Acta Neuropathol 105, 2003, pp. 586-592, PMID 12734665 .
- ↑ a b M. C. Papadopoulos u. a .: Occludin expression in microvessels of neoplastic and non-neoplastic human brain. In: Neuropathol Appl Neurobiol 27, 2001, pp. 384-395, PMID 11679090 .
- ↑ RK Jain et al. a .: Angiogenesis in brain tumors. In: Nat Rev Neurosci. 8, 2007, pp. 610-622, PMID 17643088 (review).
- ↑ KH Plate u. a .: Vascular endothelial growth factor is a potential tumor angiogenesis factor in human gliomas in vivo. In: Nature . 359, 1992, pp. 845-848, PMID 1279432 .
- ↑ J. Gavard and JS Gutkind: VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. In: Nat Cell Biol. 8, 2006 1223-1234, PMID 17060906 .
- ↑ T. Würdinger, BA Tannous: Glioma angiogenesis: Towards novel RNA therapeutics. In: Cell Adh Migr. 3, pp. 230-235, PMID 19262177 , PMC 267989 (free full text) (review).
- ↑ T. Visted et al. a .: Mechanisms of tumor cell invasion and angiogenesis in the central nervous system. In: Front Biosci. 8, 2003, pp. E289-e304, PMID 12700036 (review).
- ↑ M. Prados and C. Wilson: Neoplasms of the central nervous system. In: Cancer Medicine. Lea & Febiger publishing house, 1993, ISBN 0-8121-1422-1 , pp. 1080-1119.
- ↑ TH Lee et al. a .: Vascular endothelial growth factor modulates the transendothelial migration of MDA-MB-231 breast cancer cells through regulation of brain microvascular endothelial cell permeability. In: J Biol Chem. 278, 2003, pp. 5277-5284, PMID 12446667 .
- ↑ D. Marchetti et al. a .: Brain-metastatic melanoma: a neurotrophic perspective. (PDF file; 269 kB) In: Pathol Oncol Res. 9, 2003, pp. 147-158, PMID 14530807 (review).
- ^ BC Lee et al. a .: Involvement of the chemokine receptor CXCR4 and its ligand stromal cell-derived factor 1alpha in breast cancer cell migration through human brain microvascular endothelial cells. In: Mol Cancer Res 2, 2004, pp. 327-338, PMID 15235108 .
- ↑ N. Vykhodtseva et al. a .: Progress and problems in the application of focused ultrasound for blood-brain barrier disruption. In: Ultrasonics. 48, 2008, pp. 279-296, PMID 18511095 .