Tetrahydropyran-2-methanol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
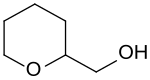
|
|||||||||||||||||||
| Structure without information on stereochemistry | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Tetrahydropyran-2-methanol | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 6 H 12 O 2 | ||||||||||||||||||
| Brief description |
clear, colorless liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 116.16 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.027 g cm −3 at 25 ° C |
||||||||||||||||||
| Melting point |
<−70 ° C |
||||||||||||||||||
| boiling point |
|
||||||||||||||||||
| Vapor pressure |
0.05 kPa at 20 ° C |
||||||||||||||||||
| solubility |
Completely soluble in water, ethanol and diethyl ether as well as in dichloromethane |
||||||||||||||||||
| Refractive index |
1.4570-1.4590 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Tetrahydropyran-2-methanol is a six-membered heterocyclic ether which has a hydroxymethyl group in the 2-position . The compound is a central intermediate stage in the synthesis of 1,6-hexanediol from the renewable raw materials glycerine as a C 3 building block or hydroxymethylfurfural as a C 6 building block.
Manufacturing
When heated with sulfuric acid to> 300 ° C at pressures> 25 MPa, glycerol is dehydrated to acrolein , which dimerizes in a [4 + 2] cycloaddition to the acrolein dimer . The catalytic hydrogenation of acrolein dimer leads to tetrahydropyran-2-methanol.
The oxidative cyclization of 5-hexen-1-ol (from 6-chlorohexanol by flash pyrolysis at 700 ° C. in 87% yield) with hydrogen peroxide in the presence of titanium silicalite zeolite gives THP-2M in a yield of 88%.
1,2,6-Hexanetriol reacts under acid catalysis with trifluoromethanesulphonic acid quantitatively with cyclization to tetrahydropyran-2-methanol.
properties
Tetrahydropyran-2-methanol is a clear, colorless liquid that dissolves completely with water and with many organic solvents , e.g. B. ethanol, diethyl ether or methylene chloride mixes.
Applications
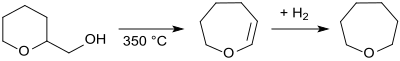
Tetrahydropyran-2-methanol can be dehydrated in the vapor phase by passing it over an aluminum silicate contact at 350 ° C. to give the vinyl ether 2,3,4,5-tetrahydrooxepine and hydrogenated over a nickel contact at 110 ° C. to give Oxepan .
Lanthanum chloride LaCl 3 -induced Michael addition of THP-2M to maleates produces the corresponding succinic acid derivative in 89% yield, which was investigated for its suitability as a complexing agent for calcium ions.
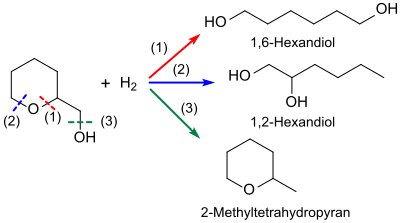
In the hydrogenation of tetrahydropyran-2-methanol, different products or product mixtures are formed, depending on the process conditions and often strongly fluctuating conversions.
Hydrogenation by means of bifunctional rhodium - rhenium -RhRe catalysts on activated carbon , Rh-RhO x / C or Rh-RhO x / SiO 2 catalysts appears to be promising for the targeted production of 1,6-hexanediol, which is of interest as a diol component in polyesters and polyurethanes . High selectivities (> 96%) for 1,6-hexanediol at relatively low conversions of 26 to 36% can be achieved in highly dilute aqueous solutions at 100-120 ° C. and reaction times of approx. 24 h. Higher conversions with longer reaction times lead to heterogeneous mixtures of substances. The disadvantage is the mostly inconsistent composition of the rhodium-rhenium catalysts and their high price.
An alternative route from tetrahydropyran-2-methanol to 1,6-hexanediol without the use of expensive noble metal catalysts has recently been reported.
THP-2M is dehydrated on zeolites to 2,3,4,5-tetrahydrooxepine with a yield of 40%, this is hydrated to 2-oxepanol and 6-hydroxyhexanal (combined yield of 85%) and quantitatively on a nickel-activated carbon Contact hydrated. The total 1,6-hexanediol yield from THP-2M is 34%.
The obtained from renewable raw materials as 1,6-hexanediol can oxidatively caprolactone are cyclized, which to the biodegradable plastic polycaprolactone or with ammonia to the more important polyamide - monomer ε-caprolactam for polycaprolactam can be implemented.
Individual evidence
- ↑ a b c d e data sheet tetrahydropyran-2-methanol 98% from Sigma-Aldrich , accessed on April 6, 2018 ( PDF ).
- ↑ Data sheet 2- (Hydroxymethyl) tetrahydropyran 94% at AlfaAesar, accessed on April 6, 2018 ( PDF )(JavaScript required) .
- ↑ a b c Entry on tetrahydropyran-2-methanol at TCI Europe, accessed on April 6, 2018.
- ^ William M. Haynes: CRC Handbook of Chemistry and Physics, 96th Edition . CRC Press, Boca Raton, FL, USA 2015, ISBN 978-1-4822-6097-7 , pp. 3-502 .
- ↑ JP Barnier, J. Champion, JM Conia: Cyclopropanecarboxaldehyde In: Organic Syntheses . 60, 1981, p. 25, doi : 10.15227 / orgsyn.060.0025 ; Coll. Vol. 7, 1990, p. 129 ( PDF ).
- ↑ a b c K. Chen, S. Koso, T. Kubota, Y. Nakagawa, K. Tomishige: Chemoselective hydrogenolysis of tetrahydropyran-2-methanol to 1,6-hexanediol over rhenium-modified carbon-supported rhodium catalysts . In: ChemCatChem . tape 2 , 2010, p. 547-555 , doi : 10.1002 / cctc.201000018 .
- ↑ K. Tomishige, Y. Nakagawa, M. Tamura: Production of Platform Chemicals from Sustainable Resources . In: Z. Fang, RL Smith, Jr., X. Qi (Eds.): Biofuels and Biorefineries . tape 7 . Springer-Verlag, 2017, ISBN 978-981-10-4171-6 , pp. 364 .
- ↑ M. Watanabe, T. Iida, Y. Aizawa, TM Aida, H. Inomata: Acrolein synthesis from glycerol in hot-compressed water . In: Bioresour. Technol. tape 98 , no. 6 , 2007, p. 1285-1290 , doi : 10.1016 / j.biortech.2006.05.07 .
- ↑ Patent US20160159715A1 : Process for producing 1,6-hexanediol. Registered on December 2, 2015 , published on June 9, 2016 , applicant: EI Du Pont de Nemours and Co., inventor: RJ Davis, CA Menning, JE Murphy, JC Ritter, SK Sengupta.
- ↑ LW Jenneskens, CAM Hoefs, UE Wiersum: Preparative flash vacuum thermolysis. Selective elimination of 6-chloro-1-hexene from esters of 6-chloro-1-hexanol with Schoenberg rearrangement of the S-methyl xanthate . In: J. Org. Chem. Band 54 , no. 24 , 1989, pp. 5811-5814 , doi : 10.1021 / jo00285a030 .
- ↑ A. Bhaumik, T. Tatsumi: Highly efficient and regio selective cyclization catalyzed by titanium silicate-1 . In: Chem. Commun. No. 4 , 1998, pp. 463-464 , doi : 10.1039 / A708528A .
- ↑ a b c T. Buntara, S. Noel, PH Phua, I. Melián-Cabrera, JG de Vries, HJ Heeres: From 5-hydroxymethylfurfural (HMF) to polymer precursors: Catalyst screening studies on the conversion of 1,2, 6-hexanetriol to 1,6-hexanediol . In: Top. Catal. tape 55 , no. 7-10 , 2012, pp. 612-619 , doi : 10.1007 / s11244-012-9839-6 .
- ↑ Patent US3636053 : Preparation of 2,3,4,5-tetrahydrooxepine. Applied on April 1, 1969 , published January 18, 1972 , applicant: Monsanto Co., inventor: DA Tyssee.
- ^ EGK Quartey, JA Peters, H. van Bekkum, T. Anthonsen: La III -induced addition of tetrahydrofurfuryl alcohol, tetrahydropyran-2-ylmethanol, D-glucose, methanol and ethanol to maleate . In: Acta Chem. Scand. tape 50 , 1996, pp. 825-831 , doi : 10.3891 / acta.chem.scand.50-0825 .
- ↑ a b J. He et al .: Production of α, ω-diols from Biomass. (PDF) In: Thermal and Catalytic Sciences 2016. University of Wisconsin-Madison, November 3, 2016, accessed April 14, 2018 .
- ↑ SP Burt, KJ Barnett, DJ McClelland, P. Wolf, JA Dumesic, GW Huber, I. Hermans: Production of 1,6-hexanediol from tetrahydropyran-2-methanol by dehydration-hydration and hydrogenation . In: Green Chem. Band 19 , 2017, p. 1390-1398 , doi : 10.1039 / C6GC0360F .
- ↑ T. Buntara, S. Noel, PH Phua, I. Melián-Cabrera, JG de Vries, HJ Heeres: Caprolactam from renewable resources: Catalytic conversion of 5-hydroxymethylfurfural into caprolactone . In: Angew. Chem. Band 123 , no. 31 , 2011, p. 7221-7225 , doi : 10.1002 / anie.201102156 .