2,5-furandicarbaldehyde
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 2,5-furandicarbaldehyde | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 4 O 3 | |||||||||||||||
| Brief description |
white to yellow crystal powder or white needles |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 124.09 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
|
|||||||||||||||
| solubility |
soluble in dimethyl sulfoxide , toluene , cyclohexane , dichloromethane |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
2,5-furandicarbaldehyde is a five-membered oxygen-containing heteroaromatic which has an aldehyde group on each of the two carbon atoms in the vicinity of the oxygen atom . The dialdehyde is produced by oxidation of the precursor 5-hydroxymethylfurfural HMF, which is the focus of intensive research as a platform chemical made from renewable raw materials. The synthesis from monosaccharides such as fructose or glucose or directly from polysaccharides such as cellulose or lignocellulose is of particular interest for industrial production . 2,5-furandicarbaldehyde has already been investigated as a building block for active pharmaceutical ingredients, ligands , bifunctional crosslinkers and monomers .
Occurrence and representation
Synthesis from 5-hydroxymethylfurfural
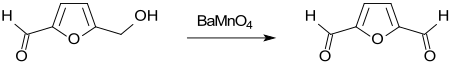
For the preparation of 2,5-diformylfuran from hydroxymethylfurfural HMF, a large number of oxidizing agents have been proposed which make the target product as quantitative as possible, i.e. H. should produce while largely avoiding the further reaction to 5-formyl-2-furancarboxylic acid FFCA and 2,5-furandicarboxylic acid FDCA. The oxidation of HMF with barium manganate BaMnO 4 in 1,1,2-trichloroethane to 2,5-diformylfuran in a yield of 93% was already described in an older publication .
In the present case of the oxidation of a heteroaromatic alcohol - especially with the high temperatures (up to> 150 ° C) and long reaction times (up to> 10 hours) that are often required - further side reactions, such as decarbonylation and polymerization , must be expected in addition to overoxidation .
The HMF oxidation at room temperature with inexpensive sodium nitrite NaNO 2 in non-toxic phosphoric acid (although only described in millimole batches ) appears interesting , with DFF being produced in practically quantitative yield within one hour. Most of the oxidizing agents and solvents used are u. a. As a rule, not for larger batches or even for industrial processes because of high costs, toxicity or complex separation.
The air or oxygen oxidation of HMF to DFF, catalyzed with noble metals or transition metal compounds, is more promising in this regard and yields in aprotic dipolar solvents such as e.g. B. dimethyl sulfoxide DMSO, dimethylformamide DMF or acetonitrile at mostly high temperatures (> 110 ° C) with quantitative conversion of HMF up to 99% 2,5-diformylfuran. However, reaction times (> 5 hours), the preparation and service life of the catalysts and the effort involved in isolating and purifying the product are still unsatisfactory. The obvious alternative with water as the reaction medium, in which the reactant hydroxymethylfurfural dissolves very well and the product furan-2,5-dicarbaldehyde hardly dissolves (<0.1 percent by weight at 25 ° C) has only been available on a laboratory scale with nanoparticulate titanium dioxide TiO 2 DFF in 88% yield.)
Synthesis from fructose
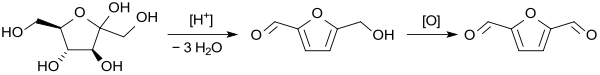
Due to the high costs due to the high isolation and cleaning effort and therefore low availability, 5-hydroxymethylfurfural is not (yet) suitable as a starting material for furan-2,5-dicarboxaldehyde. The amylase z. B. from cheap corn starch accessible monosaccharide D- glucose is isomerized on a large scale in D- fructose, which converts to hydroxymethylfurfural by triple dehydration in acid. The reaction is usually carried out in dimethyl sulfoxide, since DMSO is a good solvent for fructose (which is mainly present as five-membered β- furanose ) and suppresses side reactions of the HMF.
The HMF-containing solution is oxidized directly to DFF as a one-pot reaction without being worked up , whereby DMSO is also mostly used as the solvent. Only catalytic oxidation with oxygen or air is suitable for technically useful processes. Vanadium , iron or molybdenum compounds are mostly used as heterogeneous oxidation catalysts . The one-pot reactions are based on fructose solutions in one stage: i.e. dehydration and oxidation in parallel or in two stages: e.g. B. Dehydration under a nitrogen atmosphere and then sequential oxidation under an oxygen atmosphere .
In a one-step process, DFF could be produced with a vanadium- zeolite catalyst with complete conversion of the starting material fructose with 86% yield. In two stages, using an iron-containing Mil-88B Metal Organic Framework MOF in ethanol with a quantitative fructose conversion, DFF were generated in a yield of> 99%.
Synthesis from cellulose or lignocellulose
As with all platform chemicals derived from biomass, the cheapest starting material for the industrial production of 2,5-diformylfuran would be cellulose or, even better, lignocellulose. However, their insolubility in conventional solvents and significantly poorer DFF yields with glucose as an intermediate are enormous obstacles. The process conditions described so far for the production of the key raw material HMF, z. B. reaction temperatures> 150 ° C, reaction times of several hours, heavy metal salts such as chromium (III) chloride CrCl 3 as catalysts and reaction media , such as. B. ionic liquids are not yet suitable for large-scale processes.
properties
2,5-Diformylfuran is a crystalline substance that is obtained during synthesis as a white powder or in the form of long needles. The dialdehyde is practically insoluble in water, but soluble in many organic solvents and therefore easy to extract from mixtures of substances. The crystal needles formed during cleaning by vacuum sublimation and subsequent Soxhlet extraction are stable to oxidation.
Applications
Heating to 200 ° C in the presence of a palladium contact decarbonylates furan-2,5-dialdehyde quantitatively to furan (50%) and furfural (48% yield).
The dialdehyde DFF is being discussed as a monomer for polyimides and phenolic resins , in which it could replace the technically insignificant terephthalaldehyde or isophthalaldehyde .
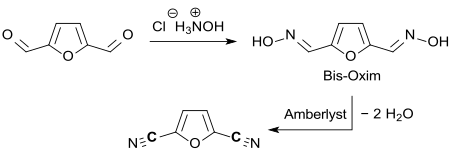
The bis- oxime of furan-2,5-carbaldehyde can be dehydrated on acidic ion exchangers in 82% yield to 2,5-dicyanofuran,
which is being discussed as a building block for active pharmaceutical ingredients or as a monomer for polymers.
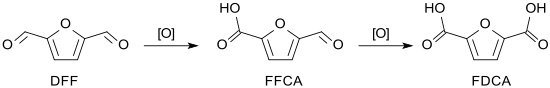
Oxidation of 2,5-diformylfuran leads via 5-formyl-2-furancarboxylic acid (FFCA) to 2,5-furandicarboxylic acid (FDCA), which is used as a substitute for terephthalic acid in the bio-based but non-biodegradable polyester polyethylene furanoate (PEF).
The commercial success of PEF, which has received great praise, is still a long way off.
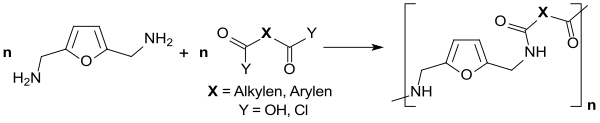
The reductive amination of DFF with ammonia NH 3 leads to the intermediate intermediate di-aldimine , which has a very strong tendency to oligomerize . With a very large excess of NH 3 , 2,5-bis (aminomethyl) furan (BAMF) can be obtained with Raney nickel in a yield of 42%.
BAMF is much better accessible via the bis-oxime on a rhodium catalyst in 94% yield.
Bio-based polyamides or polyurethanes can be produced from this primary diamine .
Other polymers with 2,5-diformylfuran as a monomer, such as. B. in polycondensates with urea , however, have no promising properties.
Individual evidence
- ↑ Entry on 2,5-diformylfuran at TCI Europe, accessed on July 27, 2019.
- ↑ a b c d Patent EP1427715B1 : Process for preparing 2,5-diformylfuran from carbohydrates. Applied September 17, 2002 , published May 17, 2006 , Applicant: EI du Pont de Nemours and Co., Inventors: V. Grushin, N. Herron, GA Halliday.
- ↑ a b c data sheet 2,5-furandicarbaldehyde from Sigma-Aldrich , accessed on July 28, 2019 ( PDF ).
- ↑ R.-J. van Putten, JC van der Waal, E. de Jong, CB .B. Rasrendra, HJ Heeres, JG de Vries: Hydroxymethylfurfural, a versatile platform chemical made from renewable resources . In: Chem. Rev. Band 113 , no. 3 , 2013, p. 1499-1597 , doi : 10.1021 / cr300182k .
- ^ T. Wang, MW Nolte, BH Shanks: Catalytic dehydration of C 6 carbohydrates for the production of hydroxymethylfurfural (HMF), as a versatile platform chemical . In: Green Chem. Band 16 , no. 2 , 2014, p. 548-572 , doi : 10.1039 / C3GC41365a .
- ↑ a b c P.Pal, S. Saravanamutugan: Recent Advances in the Development of 5-Hydroxymethylfurfural Oxidation with Base (Nonprecious) -Metal-Containing Catalysts . In: ChemSusChem . tape 12 , no. 1 , 2019, p. 145-163 , doi : 10.1002 / cssc.201801744 .
- ↑ Synthesis, chemistry and applications of 5-hydroxymethylfurfural and its derivatives; J. Lewkowski, Arkivoc , 2001 (i), 128-152 ( PDF ).
- ↑ a b T. El-Hajj, A. Masroua, JC Martin, G. Descotes: Synthèse de l'hydroxymethyl-5 furanne carboxaldehyde-2 et de ses dérivés par traitement de sucres acidic sur Resines échangeuses d'ions . In: Bull. Soc. Chim. Ms. Band 5 , 1987, pp. 855-860 , doi : 10.1134 / s1070428018030077 .
- ↑ NV Smirnova, VA Klushin, TV Bezbozhnaya, EV Khomutova, VL Lobachev, SA Mitchenko: Selective oxidation of 5- (hydroxymethyl) furfural to furan-2,5-dicarbaldehyde with sodium nitrite in phosphoric acid . In: Russ. J. Org. Chem. Volume 54 , no. 3 , 2018, p. 414-418 , doi : 10.1134 / s1070428018030077 .
- ↑ S. Gajula, K. Inthumathi, SR Arumugam, K. Srinivasan: Strategic designing on selection of solvent systems for conversion of biomass sugars to furan derivatives and their separation . In: ACS Sustain. Chem. Eng. tape 5 , no. 6 , 2017, p. 5373-5381 , doi : 10.1021 / acssuschemeng.7b00681 .
- ↑ D. Gupta, KK Pant, B. Saha: Titania nanoparticles embedded in functionalized carbon for the aqueous phase oxidation of 5-hydroxymethylfurfural . In: Mol. Catal. tape 435 , 2017, p. 182–188 , doi : 10.1016 / j.mcat.2017.03.028 .
- ↑ L. Shuai, J. Luterbacher: Organic solvent effect in biomass conversion reactions . In: ChemSusChem . tape 9 , no. 2 , 2016, p. 133-155 , doi : 10.1002 / cssc.201501148 .
- ↑ W. Zhang, W. Hou, T. Meng, W. Zhuang, J. Xie, Y. Zhou, J. Wang: Direct synthesis of V-containing all-silica beta-zeolite for efficient one-pot, one-step conversion of carbohydrates into 2,5-diformylfuran . In: Catal. Sci. Technol. tape 7 , no. 24 , 2017, p. 6050-6058 , doi : 10.1039 / C7CY01834G .
- ↑ R. Fang, R. Luque, Y. Li: Efficient one-pot fructose to DFF conversion using sulfonated magnetically separable MOF-derived Fe 3 O 4 (111) catalysts . In: Green Chem. Band 19 , no. 3 , 2017, p. 647-655 , doi : 10.1039 / C6GC02018F .
- ^ F. Menegazzo, E. Ghedini, M. Signoretto: 5-Hydroxymethylfurfural (HMF) Production from Real Biomasses . In: Molecules . tape 23 , no. 1 , 2018, p. 1-18 , doi : 10.3390 / molecules23092201 .
- ↑ Patent US20130317192A1 : Processes for preparing diacids, dialdehydes and polymers. Registered on July 25, 2013 , published on November 28, 2013 , applicant: EI du Pont de Nemours and Co., inventors: V. Grushin, LE Manzer, W. Partenheimer.
- ↑ I. Delidovich, PJC Hausoul, L. Deng, R. Pfützenreuter, M. Rose, R. Palkovits: Alternative monomer based on lignocellulosic and Their use for polymer production . In: Chem. Rev. Band 116 , no. 3 , 2016, p. 1540-1599 , doi : 10.1021 / acs.chemrev.5b00354 .
- ↑ a b Y. Xu, X. Jia, J. Ma, J. Gao, F. Xia, X. Li, J. Xu: Selective synthesis of 2,5-bis (aminomethyl) furan via enhancing the catalytic dehydration-hydrogenation of 2,5-diformylfuran dioxime . In: Green Chem. Band 20 , no. 12 , 2018, p. 2697-2701 , doi : 10.1039 / C8GC00947C .
- ↑ polyethylenes Furanoate (PEF) - The Rising Star Amongst Today's Bioplastics. Omnexus - Specialchem, July 31, 2018, accessed August 8, 2019 .
- ↑ N.-T. Le, A. Byun, Y. Han, K.-I. Lee, H. Kim: Preparation of 2,5-Bis (aminomethyl) furan by direct reductive amination of 2,5-diformylfuran over nickel-raney catalysts . In: Green and Sustainable Chemistry . tape 5 , 2015, p. 115-127 , doi : 10.4236 / gsc.2015.53015 .
- ↑ Patent WO2017005812A1 : Production of a polyamide containing 2,5-bis (aminomethyl) furan. Registered on July 6, 2016 , published on January 12, 2017 , applicant: BASF SE, inventor: GJM Habraken, JK Sprafke, M. da Silva.
- ↑ AS Amarasekara, D. Green, LD Williams: Renewable resources based polymers: Synthesis and characterization of 2,5-diformylfuran-urea resin . In: Eur. Polym. J. Band 45 , no. 2 , 2009, p. 595-598 , doi : 10.1016 / j.eurpolymj.2008.11.012 .