5-fluorouracil
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| Structural formula of the dioxo tautomer | |||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | 5-fluorouracil | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 4 H 3 FN 2 O 2 | ||||||||||||||||||
| Brief description |
White dust |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 130.08 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
282–286 ° C (decomposition) |
||||||||||||||||||
| solubility |
soluble in water (11.1 g l −1 at 22 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
5-Fluorouracil ( 5-FU ), also fluorouracil ( INN ), is a heterocyclic organic compound with a pyrimidine backbone and two carbonyl groups in positions 2 and 4 and a fluorine in position 5. It is a derivative of the nucleobase uracil .
It is a drug that is used as a cytostatic in chemotherapy , especially in colorectal cancer and breast cancer . It was developed by the American chemist Charles Heidelberger and brought onto the market in 1962 by the pharmaceutical company Hoffmann-La Roche . Decisive for the synthesis of 5-FU were Heidelberger's considerations on the tumor-inhibiting potential of fluoroacetate , the salt of fluoroacetic acid . Heidelberger later described the metabolism of 5-FU with reference to the lethal synthesis principle ( biotransformation of fluoroacetate to fluorocitrate) developed by the British biochemist and warfare agent researcher Sir Rudolph Peters .
In small doses and in combination with active ingredients with salicylic acid , 5-fluorouracil is also used for external application as a wart therapeutic agent under trade names such as Verrumal ® .
properties
5-fluorouracil is a solid that melts at 282–286 ° C with decomposition.
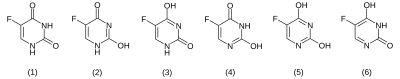
There are six possible tautomeric forms of 5-fluorouracil in the solid state, with the dioxo form (1) being preferred.
Pharmacological properties
effect
5-fluorouracil is an antimetabolite which during cell division due to the structural similarity with the pyrimidine bases cytosine and thymine ( DNA - nucleotides ) or uracil ( RNA instead-nucleotide) of these in the DNA and RNA is incorporated. The enzyme UMP pyrophosphorylase ( EC 2.4.2.9 ) converts 5-fluorouracil into 5-fluoro-UMP, which is then further phosphorylated to 5-fluoro-UTP and incorporated into the RNA . This causes the synthesis of defective RNA, whereby the protein biosynthesis is inhibited.
In addition, 5-fluorine-dUMP also inhibits thymidylate synthase ( EC 2.1.1.45 ), which ultimately leads to DNA synthesis and cell division being inhibited.
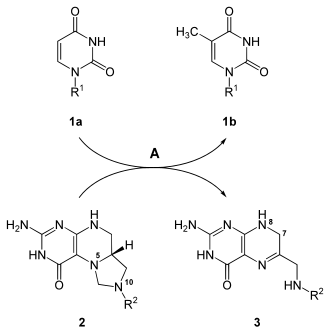 The synthesis of dTMP ( 1b ) from dUMP ( 1a ) is catalyzed by thymidylate synthase ( A ), during which N 5 , N 10 -methylene tetrahydrofolate ( 2 ) is converted to 7,8-dihydrofolate ( 3 ). 5-Fluor-dUMP inhibits the enzyme and thus this reaction.
The synthesis of dTMP ( 1b ) from dUMP ( 1a ) is catalyzed by thymidylate synthase ( A ), during which N 5 , N 10 -methylene tetrahydrofolate ( 2 ) is converted to 7,8-dihydrofolate ( 3 ). 5-Fluor-dUMP inhibits the enzyme and thus this reaction.
It is particularly effective in the interphase of the cell cycle. In addition to inhibiting DNA and RNA synthesis, it also inhibits the so-called exosome complex, which is vital for the cell.
5-fluorouracil is administered in the form of the prodrugs capecitabine or 5-fluorocytosine and only converted into the active metabolite in the cell. For example, 5-fluorocytosine is absorbed into the cell by a cytosine permease and is immediately deaminated there by the cytosine deaminase to form 5-fluorouracil .
Effect depends on genetics
According to a study, the effectiveness depends on the genetics of the patient. The single nucleotide polymorphism rs2612091 then determines in locally advanced gastric cancer essential survival. The variant A / A has a good effectiveness, the variants A / G and G / G are associated with significantly lower effectiveness. It is conceivable that this can also be transferred to other cancers. Genetic testing e.g. B. in the form of genotyping is currently not standard in treatment.
Side effects
The side effects (nausea, vomiting, inflammation of the mucous membranes, bone marrow damage) can be considerable (see the section on side effects in the article cytostatic ).
In animal experiments and patient studies, brain damage caused by damage to glial cells was identified as long-term side effects . Neuro- and cardiotoxic side effects are attributed to the 5-FU metabolite fluoroacetate.
The additional administration of tetrahydrofolic acid or folinic acid enables a higher dosage of 5-FU; this effect is used for combination therapies. Interferon-α also increases the effect .
The degradation of 5-fluorouracil takes place via the enzyme dihydropyrimidine dehydrogenase . In patients who are affected by the rare hereditary metabolic disease dihydropyrimidine dehydrogenase deficiency (DPD), treatment with 5-fluorouracil can lead to extremely severe intoxication. Affected people are often asymptomatic. The diagnosis of exclusion is based on the analysis of the purines and pyrimidines and the specific test "Exclusion of DPD in planned 5-fluorouracil therapy" from urine.
The Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency (EMA) recommends that patients should be tested for the absence or partial deficiency of DPD before starting cancer treatment with medicinal products containing fluorouracil administered by injection or infusion (drip) administered. This recommendation applies accordingly to treatment with the related active ingredients capecitabine and tegafur , which are converted into fluorouracil in the body ( prodrug ).
See also
- FOLFOX therapy regimen
- FOLFIRI therapy regimen
Trade names
Benda-5 FU (D), Efudix (D, CH), Haemato-fu (D), Neofluor (D), Onkofluor (D), Ribofluor (D), various generics (D, A, CH)
Verrumal (D, A, CH)
Individual evidence
- ↑ a b c data sheet 5-fluorouracil from Sigma-Aldrich , accessed on December 2, 2013 ( PDF ).
- ↑ a b c Entry on 5-fluorouracil in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ a b Entry on 5-fluorouracil in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ^ Charles Heidelberger: Fluorinated Pyrimidines and Their Nucleosides . In: Antineoplastic and Immunosuppressive Agents (= Handbuch der experimental Pharmakologie / Handbook of Experimental Pharmacology ). Springer, Berlin, Heidelberg, 1975, ISBN 978-3-642-65808-2 , pp. 193-231 , doi : 10.1007 / 978-3-642-65806-8_12 .
- ↑ Katherine Elliott, Joan Birch (Ed.): Carbon-Fluorine Compounds. Chemistry, Biochemistry and Biological Activities. Associated Scientific Publishers, Amsterdam 1972, ISBN 978-0-470-71985-5 , pp. 130 .
- ↑ Joachim Morschhäuser: Resistances and Resistance Mechanisms: How Do Fungi Escape Therapy? in: Pharmazie in our Zeit , 2003 , 32 (2), pp. 124-129 ( doi: 10.1002 / pauz.200390029 ).
- ^ F. von Bruchhausen: Hager's Handbook of Pharmaceutical Practice: Drugs AK , 5th edition, Springer Verlag, Berlin 1998, ISBN 3-540-61618-7 , p. 226.
- ↑ D. Meulendijks, EA Rozeman, A. Cats, K. Sikorska, M. Joerger: Pharmacogenetic variants associated with outcome in patients with advanced gastric cancer treated with fluoropyrimidine and platinum-based triplet combinations: a pooled analysis of three prospective studies . In: The Pharmacogenomics Journal . tape 17 , no. 5 , October 2017, ISSN 1473-1150 , p. 441–451 , doi : 10.1038 / tpj.2016.81 ( nature.com [accessed March 31, 2020]).
- ↑ Ruolan Han, Yin M Yang, Joerg Dietrich, Anne Luebke, Margot Mayer-Pröschel, Mark Noble: Systemic 5-fluorouracil treatment causes a syndrome of delayed myelin destruction in the central nervous system. In: Journal of Biology. 7, 2008, p. 12, doi : 10.1186 / jbiol69 .
- ↑ Harold Koenig: Biochemical Basis for Fluorouracil Neurotoxicity . In: Archives of Neurology . tape 23 , no. 2 , August 1, 1970, p. 155 , doi : 10.1001 / archneur.1970.00480260061008 ( jamanetwork.com [accessed April 30, 2018]).
- ^ H. Koenig, A. Patel: The acute cerebellar syndrome in 5-fluorouracil chemotherapy: a manifestation of fluoroacetate intoxication . In: Neurology . tape 20 , no. 4 , April 1970, p. 416 , PMID 5535078 .
- ↑ Kazumasa Yamashita, Hideaki Yada, Toshihiko Ariyoshi: Neurotoxic effects of alpha-fluoro-beta-alanine (FBAL) and fluoroacetic acid (FA) on dogs . In: The Journal of Toxicological Sciences . tape 29 , no. 2 , May 2004, p. 155-166 , PMID 15206584 .
- ↑ M. Arellano, M. Malet-Martino, R. Martino, T. Spector: 5-Ethynyluracil (GW776): effects on the formation of the toxic catabolites of 5-fluorouracil, fluoroacetate and fluorohydroxypropionic acid in the isolated perfused rat liver model . In: British Journal of Cancer . tape 76 , no. 9 , 1997, pp. 1170-1180 , PMID 9365165 , PMC 2228116 (free full text).
- ↑ M. Arellano, M. Malet-Martino, R. Martino, P. Gires: The anti-cancer drug 5-fluorouracil is metabolized by the isolated perfused rat liver and in rats into highly toxic fluoroacetate. In: British Journal of Cancer . tape 77 , no. 1 , 1998, p. 79-86 , PMID 9459149 , PMC 2151255 (free full text).
- ↑ S. Maurer, J. Thödtmann: The mammary carcinoma: diagnosis and therapy. Govi-Verlag, Eschborn 2003, ISBN 978-3-7741-0996-4 .
- ↑ Van Kuilenburg ABP (2004) Dihydropyrimidine dehydrogenase and the efficacy and toxicity of 5-fluorouracil. Eur J Cancer 40: 939-950.
- ↑ Fluorouracil, Capecitabine, Tegafur and Flucytosine: Recommendation for testing and treatment , risk assessment procedure of the BfArM, notification of March 16, 2020, accessed on March 18, 2020