Grotto olm
| Grotto olm | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
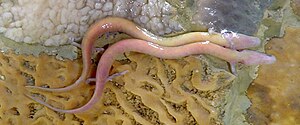
Grotto olms ( Proteus anguinus ) |
||||||||||||
| Systematics | ||||||||||||
|
||||||||||||
| Scientific name of the genus | ||||||||||||
| Proteus | ||||||||||||
| Laurenti , 1768 | ||||||||||||
| Scientific name of the species | ||||||||||||
| Proteus anguinus | ||||||||||||
| Laurenti , 1768 |
The cave salamander ( Proteus anguinus ) is a permanently living in larval form in cave waters European salamander and the only way the genus Proteus . This genus together with the North American furrows pigging the family of Olme (Proteidae). Similarities and convergent developments to the olm can also be found in some cave-dwelling lungless salamanders , such as the Texan well newt ( Eurycea rathbuni ).
features
The olm has an eel-like elongated body that can reach a length of 25 to 30 centimeters (in individual cases up to 40 centimeters). The oar tail is flattened on the sides and has fin hems. The limbs are very thin and reduced - there are only three fingers on each of the front legs and two toes on each of the hind legs. The skin is yellowish-white, looking pinkish-flesh-colored through translucent blood vessels, often with dark spots; dark pigmentation can occur when exposed to light.
The head is oblong-narrow and spatulate in front, the eyes of the nominate form Proteus anguinus anguinus have degenerated into a lack of function and are hidden under the skin of the body. The breathing takes place through the lungs ; In addition, grotto olms have three pairs of outer, red gill tufts on the back of their heads. This phenomenon, that sexually mature, reproductive animals also show and retain larval characteristics, is called neoteny or pedomorphosis. Neoteny occurs in many tail amphibians at least occasionally or temporarily; in cave olms, as in axolotl , it is obligatory. A complete metamorphosis would probably not bring any evolutionary advantage, at least within the cave habitats . Both sexes have a thickened cloacal region when sexually mature , which means that animals ready to reproduce are clearly recognizable. The swelling is more pronounced in the male than in the female; in females the eggs can usually be seen through the skin shimmering through.
Black cave olms in southern Slovenia
In 1986, a single aboveground population of cave olms was discovered in two neighboring springs in Jelševnik near Črnomelj , in the Bela krajina region (south-east Slovenia). The animals differ from the nominate form by the strong, black pigmentation, developed eyes, a longer trunk, a wider skull with a shorter ploughshare leg (vomer). They were described as a separate subspecies Proteus anguinus parkelj . The Slovenian name "parkelj" is one of the names of the (Christian) devil , also known as " Krampus ", the black mythical figure with a red tongue known from the carnival.
However, the status of the population as a subspecies is controversial. It could either be a relic form similar to the ancestral form of the grotto olms still living above ground and would then be easily characterizable as a subspecies or a re-adaptation of cave olms with secondary immigration into an exposed habitat. After an analysis of the relationships based on the sequence of the mitochondrial DNA , the surface shape cannot be differentiated from other populations, but is grouped with other, typical, populations of the geographic region. It has also been known for a long time that cave olms can adapt to light and develop a dark pigmentation with (careful and slowly increased) exposure (so they are not, as is often assumed inaccurately, albino-like ). The Austrian researcher Paul Kammerer also reported back in 1912 that he had succeeded in stimulating the rudimentary and normally functionless eyes to functional differentiation under red light irradiation. Accordingly, there is the possibility that normal, dark-adapted cave olms still have the genetic potency to adapt to these conditions when immigrating to exposed habitats. If this scenario were true, the new subspecies parkelj would make the typical subspecies paraphyletic ; this would then not be justified.
distribution
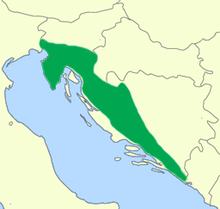
The olm lives exclusively in the Dinaric Karst , in the limestone mountains east of the Adriatic . The distribution ranges from the extreme north-east of Italy (in the catchment area of the Isonzo / Soča river with its absolute northern border in Gradisca d'Isonzo ) through Slovenia and western Croatia (including the Istrian peninsula ) to Trebišnjica in Herzegovina . Some of the populated waters drain to the Adriatic, others via the Sava and Danube to the Black Sea. However, this is sometimes difficult to determine, because the area mainly drains underground and numerous bodies of water disappear underground in ponors . The deposit furthest inland is somewhat isolated from the others in Lusci Polje in the Bosnian Krajina. Since the species enjoys considerable public attention and newly reported occurrences are usually immediately and intensively checked, the discovery of completely new occurrences of the species is considered unlikely. It is assumed, however, that the essential habitat for humans are inaccessible fissures in the karst rock. Finds from larger caves or springs accessible to humans are presumably only marginal occurrences or occurrences washed out by flood water. As the occurrence of the above-ground, darkly pigmented population proves, they can also live here permanently under favorable circumstances. The altitude distribution is difficult to specify because of the underground way of life. None of the Italian populations live higher than 20 m above sea level. NN , but partly under the karst ridges more than 300 meters below the surface.
For a long time there have been attempts by humans to relocate cave olms outside of their natural range in caves. In addition to the occurrence in Moulis in the French Pyrenees, the population in Oliero Valstagna in the Italian province of Vicenza goes back to a release in the 19th century. Most of the time, however, there was no reproduction.
Grotto olms in the Hermannshöhle
In Germany, the Hermannshöhle in the Harz near Rübeland ( Saxony-Anhalt ) is known for its grotto olms. A total of 18 specimens from Istria were released there in 1932 (5 pieces) and 1956 (13 pieces) for display purposes in an artificial cave water that has a depth of around 80 centimeters and a permanent water temperature of 7 ° C. This so-called "Olmensee" is a tourist attraction. In 1978 a breeding tank was built. An inventory made on the occasion could only identify males; in 1985 all 13 specimens recorded appeared to be male. In 2015 only 9 animals were found. Of these, however, 5 were females who also carried eggs. For the first time in 85 years, 4 eggs were found in 2016, which were transferred to separate tanks so that they can develop without interference. It is not uncommon for grotto olms to eat their own eggs. At first it was assumed that if the eggs were fertilized, young animals would already be found in May or June 2016. At the beginning of June, the responsible project manager announced that hatching would be delayed because the cave is far to the north and this slows down the development of the animals. In fact, all of the eggs died. Another ten eggs were discovered and separated in August 2017. These also died. In 2020, in a population of 7 specimens, 4 fertilized eggs were found in the fallopian tube in one of the 4 females. At the end of August 2020, two clutches with eggs were discovered.
Habitat, way of life
The species lives in flooded parts of caves ( called siphons by speleologists ), rarely also in karst springs fed by such cave waters or in open cave lakes. When using the karst groundwater, they are sometimes pumped up, and there are (unconfirmed) old reports that they occasionally migrate from the cave waters into springs and surface waters at night. Grotto olms can both breathe air and meet their oxygen requirements in the water through gills and skin breathing; when kept in terrariums, they sometimes leave the water voluntarily, even for longer periods of time, they can even hunt here. The animals look for hiding places in crevices or under stones, but never bury themselves. They always return to familiar hiding spots, which they recognize by their smell; In the experiment, at least sexually inactive animals preferred hiding places that were already occupied by conspecifics, so they are sociable. The activity of the species is appropriate to the subterranean habitat, neither diurnal nor annual; even young animals can be found equally in all seasons. Grotto olms can perceive light through a skin light sense, although their eyes are inoperative. If individual parts of the body are exposed more strongly, they flee away from the light (negative phototaxis ). However, you can get used to constant light stimuli and are even attracted by extremely weak exposure. You can also use a magnetic sense to orientate yourself in the living space.
There are partly contradicting statements about the preferred habitat of the species. While some researchers assume a preference for particularly deep, undisturbed parts of water with constant environmental conditions, others suspect a preference for areas with inflowing surface water, because the food supply is much better here. As far as is known, they hunt small crustaceans that can be found unspecifically in their habitat, such as water lice , amphibians ( Gammarus and the cave shrimp Niphargus ) and the small cave-living freshwater shrimp Troglocaris , as well as worms ( oligochaetes ). In the experiment they are not very picky and eat everything that can overwhelm them. Little is known about the natural enemies of the Olm. Wolfgang Briegleb suspects that the species cannot live in waters where crayfish are found. A specialized parasite, Chloromyxum protei Joseph , 1905 ( Myxozoa ), which parasitizes in the kidney, has so far only been identified from this species.
The olm is relatively sensitive to temperature. A comparison of water bodies it has colonized shows that (with very rare exceptions) it only colonizes bodies of water warmer than 8 ° C and prefers those above 10 ° C, although it can tolerate lower temperatures, including ice, for shorter periods of time. Water temperatures of up to around 17 ° C are tolerated without problems, even warmer water only for a short time. The development of eggs and larvae is no longer possible above 18 ° C. In groundwater and in cave waters, apart from inflowing surface water, the temperature is almost constant over the course of the year and roughly corresponds to the annual mean temperature at this location. It is possible that its distribution is limited by isotherms both in height and to the north . Although the populated waters are mostly more or less saturated with oxygen, the olm tolerates a wide range of values and can even survive a complete lack of oxygen, known as anoxia , for up to 12 hours.
Reproduction and development
Due to its way of life in fissures in the karst rock, it has not been possible until today to study the development of the cave olm in its natural habitat. Eggs have never been found in the actual habitat to date, and even younger larval stages only extremely rarely. When eggs are only observed in a karst spring, it can be assumed that they have been carried over by flood water. In the famous Postojna show cave , from which the species has been known for over 100 years, eggs were found for the first time in 2014 in the show aquarium in the visitor area, and two animals were observed hatching at the end of May 2016.
In addition to aquarium observations, data from the artificially established population from the cave of Moulis, canton Saint-Girons in the French Pyrenees, where data on the population have been documented since 1958, are available for the development of the Olm. According to this, females only reach sexual maturity at an average age of 15 to 16 years and even then only reproduce rarely, in Moulis every 12.5 years. If wild-caught animals are kept in the aquarium, a relatively large number of animals reach sexual maturity within a few months, which is associated with better nutrition. In the habitat, males occupy courtship areas (in the aquarium) about 80 centimeters in diameter, the edge of which they patrol continuously. If other males who are ready to mate come into this courtship area, violent territorial fights ensue, with the territory owner attacking the rival with bites; this can certainly cause wounds or gills to be bitten off. Animals that are not sexually mature are tolerated in the area. The animals can only recognize the sex and reproductive status of conspecifics through direct physical contact. If a sexually mature female swims into the territory, the male circled it with tail movements. Finally, the male deposits a spermatophore on the bottom of the water . The female strokes it with her cloaca and in doing so takes in sperm. This sequence can take place several times in a row. Finally the female leaves the courtship area and looks for a place to hide. Here or in its vicinity it then occupies a spawning area, the borders of which it also defends against intruders with bites. Much larger conspecifics are also attacked. The laying of the eggs, which are around 4 millimeters in size, begins around 2 to 3 days later and often takes a few weeks. The clutch size for moulis is given as 35 eggs, of which around 40 percent hatched. In the aquarium, a female laid about 70 eggs over a period of 3 days. The female defends the spawning area with the young even after they hatch. Unguarded eggs and young larvae are easily eaten by other Olmen. The larvae begin their active life with a body length of around 31 millimeters; embryonic development takes 180 days. The larvae differ from full-grown olms by their compact, rounded body shape, the smaller rear extremities and the wider fin edge, which extends forward to over the trunk. The adult body shape is reached after 3 to 4 months, the animals are then about 4.5 centimeters long.
With a life expectancy of over 70 years (determined under semi-natural conditions), some researchers even assume 100 years, the species can become many times older than is generally common in amphibians . In the population in Moulis, which was kept under semi-natural conditions, animals (which had been caught in the wild of unknown age) were sometimes observed for over 48 years of life without senescence or a decrease in life expectancy in the older animals . The researchers use their life table data (regression degrees) to calculate a generation duration of 36.5 years and an average life expectancy of 68.5 years for animals in their sixth year of life (i.e. without taking youth mortality into account). The high life expectancy is extraordinary for an animal with the low body mass of the oil (around 20 grams) and has not yet been fully scientifically explained. The cave habitat certainly plays a role with its unfavorable but highly predictable and uniform conditions.
Some researchers have published observations according to which the cave olm would wean live young or they would hatch immediately after oviposition ( viviparia or ovoviviparia). However, on closer examination, eggs were always laid. It is possible that these observations go back to animals kept under extremely unfavorable conditions.
Research history
In earlier times, the cave olm was thought to be a dragon cub because of its exterior. Because of its skin-like body color, it is also called “human fish ” - this is the translation of its name into Croatian ( čovječja ribica ), Serbian ( Човечја рибица ) and Slovenian ( človeška ribica ) language. A font from the church of San Nicolò in Venice from the 10th or 11th century, which is now kept in the Kunsthistorisches Museum in Vienna, shows two elongated animals, which could be grotto olms.
The first published description of the cave olm is the report by Johann Weichard von Valvasor from 1689. He had learned from a farmer that a " lindworm " lived underground in the Bela valley near Vrhnika . When doubts were expressed, the farmer appealed to the postmaster Hofmann zu Ober-Laybach. After the postmaster's visit and his own examination of the animal, Valvasor remarks that it was "shaped like an Eydexen". Later editors point out that the location mentioned by Valvasor is implausible and presumably a false statement. The first actual discovery of an animal in a cave was made by Josip Jeršinovič Knight von Löwengreif, district treasurer in Adelsberg ( Postojna ), in the "Magdalene Cave " (the Črna Jama, part of the Postojna Caves ) in 1797, later set as the type locality for the species . The first scientific description of the species by Joseph Nicolai Laurenti in 1768 made this after animals from Stična , which Giovanni Antonio Scopoli had sent him. It was the first description of a purely cave-living (technical term troglobiont ) animal species worldwide. The find caught the imagination of some of the leading naturalists of their time: the fossils of fossilized reptile bones found at the time were attributed to cave-living Proteus relatives by Georges Cuvier or William Kirby (who, for theological reasons, assumed that species could not become extinct , and explained the absence living specimens with their hidden, underground way of life). In the 19th century cave olms in the Postojna Caves were sold to visitors as curiosities and souvenirs; A number of live animals came to Northern Europe through travelers, including well-known ones such as Charles Babbage , William John Hamilton or Francis Galton , where the animals were shown, for example, in the London Zoo .
Hazard and protection
Danger
The cave olm is listed by the IUCN in the red list in the category "vulnerable" (vu) (cf. under web links). The main sources of danger are surface water pollution of the underground karst waters, also through intensified land use or urbanization, and use of groundwater as industrial or drinking water as well as use of water to generate electricity. Other causes such as direct persecution or tourist expansion of caves are only of minor importance. Because of the high paths in the fissured rock, karst groundwater is particularly sensitive to pollution.
In the center of the Grottenolm area, for example, is the Iskra factory for the production of capacitors for the electrical industry, which is held responsible for the massive groundwater pollution in the Krupa catchment area (see article PCB pollution of Krupa ). The poisonous polychlorinated biphenyls are also accumulated in the fatty tissue of grotto olms from the region. But the arsenic concentration in the tissue of many olms is also greatly increased, which is attributed to arsenic-containing pesticides , especially from viticulture. Although the olms appear to survive the acute concentrations, little is known about possible chronic effects. The population size and a possible population trend are not known for the species in any part of its area, the reason is the difficult and coincidentally dependent detection of the species. The decline in the Italian and Slovenian occurrences is therefore suspected for good reasons, but is not linked to population figures underpin. It is known from Slovenia that some deposits in Kočevsko polje (the largest karst plain in Slovenia) in the Kočevje region (formerly Gottschee) were extinct due to industrial wastewater and from a landfill. In addition, there are no reliable findings.
Species protection
In the European Union, the species is a “ species of community interest ”. It is listed in the Fauna-Flora-Habitat Directive in Annexes II and IV. For species listed in Appendix II , the member states must designate special protected areas that are to become part of the Natura 2000 protected area system. The olm is one of the "priority" species because the EU has a special responsibility for its survival. Species in Appendix IV, including their habitats, are also particularly protected wherever they occur. In the case of projects and interventions in nature that can affect the population, it must be proven in advance that they do not threaten the population - even outside of protected areas. The protection categories of the Habitats Directive apply directly across the EU and are generally included in national legislation, including in Germany. The olm is also protected in Croatia, Slovenia and Italy; in Slovenia, animal trade has been banned since 1982. The most significant occurrences of the Olm cave in Slovenia are now covered by Natura 2000 protected areas, although some populations are still considered endangered.
Phylogeny, kinship, evolution
The genus Proteus with the grotto olm as the only species is united with the American genus Necturus in the family of the Proteidae. The summary based on morphological similarities has long been considered uncertain and has been repeatedly disputed. Phylogenomic examinations based on homologous DNA sequences predominantly support the togetherness, even if in some cases not with outstanding statistical certainty. Using the methods of the molecular clock , a split of the lines in the Lower Cretaceous is revealed; at this point in time the supercontinent Laurasia was not yet completely split into Eurasia and North America. The current occurrence is therefore probably a relic of what was once a wider distribution.
Richard Estes and Ilya Darevsky have described a fossil salamander from the Miocene of the Caucasus as Mioproteus caucasicus , which could belong to the tribe of the recent grotto olms. Because of the proportions of the body, they assume neoteny for him too. In contrast to the recent grotto olm, it still had a stable and ossified spine and ribs. However, features of the dentition make it unlikely that he himself could have been an ancestor of the Olm. Due to the circumstances of the find and the accompanying fauna, they adopt surface water, probably a lake, as their habitat. There are no convincing fossil finds of Proteus itself or possible extinct relatives (Batrachosauroididae) from the Dinarides.
The origin of today's area of the Olm Grotto cannot yet be explained satisfactorily. The Dinarides have probably been mainland since the Oligocene . In today's area of the Olm Grotto, however, it must have been too cold for the eggs and larvae to develop successfully during the Ice Age. Proteus does not occur in regions that were glaciated in the Pleistocene , but the northernmost occurrence is only about 30 kilometers south of the extreme southern ice edge. It is possible either that the species from refuges further south did not colonize the region until the post-glacial period, or that it was still living above ground at that time. Direct sunlight makes development more likely in above-ground waters than in cave waters, even if the average temperatures are the same.
Others
The Slovenian city of Postojna has an almost realistically drawn cave olm in its coat of arms.
literature
- Constantin von Wurzbach : Petsch Ritter von Löwengreif, Joseph . In: Biographisches Lexikon des Kaiserthums Oesterreich . 24th part. Imperial and Royal Court and State Printing Office, Vienna 1872, p. 129 ( digitized version ).
- Dieter Glandt: Pocket dictionary of amphibians and reptiles in Europe. Quelle & Meyer, Wiebelsheim 2010, ISBN 978-3-494-01470-8 .
- Václac Laňka, Zbyšek Vít: amphibians and reptiles. Translated from the Czech by Emma Echsnerová. Artia, Prague 1984, pp. 52-53.
- Andreas Nöllert, Christel Nöllert: The amphibians of Europe . Franckh-Kosmos, Stuttgart 1992, ISBN 3-440-06340-2 .
Web links
- Article about grotto olms from the FAZ Sunday newspaper (PDF file; 24 kB)
- Proteus anguinus at Amphibiaweb.org
- Proteus anguinus at Amphibian Species of the World 6.0, an Online Reference
- Proteus anguinus at EDGE
- Devonkarst.org: The Proteus Project
- Proteus anguinus in the endangered Red List species the IUCN 2009. Posted by: Arntzen et al. , 2008.
Individual evidence
- ↑ Boris Sket, Jan Willem Arntzen: A black, non-troglomorphic amphibian from the karst of Slovenia: Proteus anguinus parkelj n. Ssp. (Urodela: Proteidae). In: Bijdragen tot de Dierkunde. 64 (1), 1994, pp. 33-53.
- ↑ Ana Ivanović, Gregor Aljančič, Jan W. Arntzen: Skull shape differentiation of black and white olms (Proteus anguinus anguinus and Proteus a. Parkelj): an exploratory analysis with micro-CT scanning. In: Contributions to Zoology. 82 (2), 2013, pp. 107-114.
- ↑ Špela Gorički, Peter Trontelj: Structure and evolution of the mitochondrial control region and flanking sequences in the European cave salamander Proteus anguinus. In: Genes. 378, 2006, pp. 31-41. doi: 10.1016 / j.gene.2006.04.016
- ↑ a b R. S. Hawes: On the eyes and reactions to light of Proteus anguinus. In: Quarterly Journal of Microscopical Science. 86, 1945, pp. 1-53.
- ^ Peter Berz: The Eyes of the Olms. In: History and Philosophy of the Life Sciences. 31, 2009, pp. 215-238.
- ↑ a b c d e Nicola Bressi: Underground and unknown: updated distribution, ecological notes and conservation guidelines on the Olm Proteus anguinus anguinus in Italy (Amphibia, Proteidae). In: Italian Journal of Zoology. 71, Supplement 1, 2004, pp. 55-59.
- ↑ a b c d Boris Sket: Distribution of Proteus (Amphibia: Urodela: Proteidae) and its Possible Explanation. In: Journal of Biogeography. Vol. 24, No. 3, 1997, pp. 263-280. (Access via JSTOR )
- ↑ Wolf-Rüdiger Grosse: Grottenolm - Proteus anguinus Laurenti, 1768. In: Frank Meyer et al. (Ed.): The amphibians and creeping animals of Saxony-Anhalt. Laurenti-Verlag, Bielefeld 2004, ISBN 3-933066-17-4 , pp. 191-193.
- ↑ Among the Olmen of the Hermannshöhle are females. In: Volksstimme . January 22, 2015, accessed September 10, 2015.
- ↑ Sensation: Grotto olms have laid eggs. In: Volksstimme. February 26, 2016. Retrieved February 26, 2016.
- ↑ a b Something is stirring at the olm. In: Hamburger Abendblatt. March 24, 2016, p. 21.
- ↑ Legendary olms visible to everyone . In: Volksstimme. July 10, 2016, accessed September 11, 2016.
- ↑ Again no offspring for Harz cave olms. ndr.de, October 27, 2017, accessed April 25, 2018 .
- ↑ Hope again for young olms in the stalactite cave. rtl.de, June 6, 2020, accessed June 12, 2020 .
- ↑ Hope of offspring: fertilized olm eggs found
- ↑ a b c d e f g Wolfgang Briegleb: On the biology and ecology of the grotto olms (Proteus anguinus Laur. 1768). In: Journal for Morphology and Ecology of Animals. 51, 1962, pp. 271-334.
- ↑ O. Guillaume: Role of chemical communication and behavioral interactions among conspecifics in the choice of shelters by the cave-dwelling salamander Proteus anguinus (Caudata, Proteidae). In: Canadian Journal of Zoology. 78, 2000, pp. 167-173.
- ↑ a b Peter A. Schlegel, Sebastian Steinfartz, Boris Bulog: Non-visual sensory physiology and magnetic orientation in the Blind Cave Salamander, Proteus anguinus (and some other cave-dwelling urodele species). Review and new results on light-sensitivity and non-visual orientation in subterranean urodeles (Amphibia). In: Animal Biology. 59, 2009, pp. 351-384. doi: 10.1163 / 157075609X454971
- ↑ H. Joseph: Chloromyxum protei n. Sp. In: Zoologischer Anzeiger. 29, 1905, pp. 450-451. Full text source via Biostor
- Jump up ↑ Jorge C. Eiras: An overview on the myxosporean parasites in amphibians and reptiles. In: Acta Parasitologica. 50 (4), 2005, pp. 267-275.
- ↑ J. Issartel, F. Hervant, M. de Fraipont, J. Clobert, Y. Voituron: High anoxia tolerance in the subterranean salamander Proteus anguinus without oxidative stress nor activation of antioxidant defenses during reoxygenation. In: Journal of Comparative Physiology. B 179 (4), 2009, pp. 543-551. doi: 10.1007 / s00360-008-0338-9 . tested at 12 ° C
- ↑ B. Sket, F. Velkovrh: The discovery of Proteus eggs (Proteus anguinus Laurenti, Amphibia) in seminatural conditions. In: International Journal of Speleology. 10, 1978, pp. 205-209.
- ↑ Miracle in Postojna Cave Aquarium - Proteus anguinus laying eggs ( Memento from August 21, 2014 in the Internet Archive )
- ↑ "The Birth of the Dragons". Retrieved June 13, 2016 .
- ↑ a b Yann Voituron, Michelle de Fraipont, Julien Issartel, Olivier Guillaume, Jean Clobert: Extreme lifespan of the human fish (Proteus anguinus): a challenge for aging mechanisms. In: Biological Letters. 7 (1), 2010, pp. 105-107. doi: 10.1098 / rsbl.2010.0539
- ↑ Jakob Parzefall: Behavioral ecology of aquatic salamanders colonizing subterranean habitats. In: Proceedings of the 15th International Congress of Speleology. 2009, pp. 270-273.
- ↑ Ronald A. Nussbaum: Parental care and egg size in salamanders: an examination of the safe harbor hypothesis. In: Research in Population Ecology. 29, 1987, pp. 27-44.
- ↑ Researchers are puzzling over the old age of the Grotto Olm. In: Spiegel online. July 21, 2010.
- ^ Nelson G. Hairston: Community Ecology and Salamander Guilds. Cambridge University Press, 1987, ISBN 0-521-32578-1 , p. 85.
- ^ A b Trevor R. Shaw: Proteus for sale and for science in the 19th Century. In: Acta Carsologica. 28 (1), 1999, pp. 229-304.
- ^ Irmgard Palladino, Maria Bidovec: Johann Weichard von Valvasor (1641–1693). A protagonist of the early modern science revolution. Life, work and estate. Böhlau Verlag, Vienna 2008, ISBN 978-3-205-77719-9 , p. 64.
- ^ Stephan Kempe, Hans-Peter Hubrich: Inscriptions of some historically known persons in Postojnska jama. In: Acta Carsologica. 49 (2), 2011, pp. 397-415.
- ↑ Marko Pezdirc, Ester Heath, Lilijana Bizjak Mali, Boris Bulog: PCB accumulation and tissue distribution in cave salamander (Proteus anguinus anguinus, Amphibia, Urodela) in the polluted karstic hinterland of the Krupa River, Slovenia. In: Chemosphere. 84, 2011, pp. 987-993. doi: 10.1016 / j.chemosphere.2011.05.026
- ↑ Boris Bulog, Katarina Mihajl, Zvonka Jeran, Mihael J. Toman: Trace element concentrations in the tissues of Proteus anguinus (Amphibia, Caudata) and the surrounding environment. In: Water, Air, and Soil Pollution. 136, 2002, pp. 147-163.
- ↑ a b Andrej Hudoklin: Are we guaranteering the favorable status of the Proteus anguinus in the Natura 2000 Network in Slovenia? In: Mitja Prelovšek, Nadja Zupan Hajna (eds.): Pressures and Protection of the Underground Karst. Cases from Slovenia and Croatia. Založba ZRC, 2011, ISBN 978-961-254-285-6 , pp. 169-181. Preview on Google Books
- ↑ Grottenolm at www.wisia.de
- ↑ Peter Trontelj, Spela Goricki: Monophyly of the family Proteidae (Amphibia: Caudata) tested by phylogenetic analysis of mitochondrial 12s rDNA sequences. In: Natura Croatica. Vol. 12, No. 3, 2003, pp. 113-120.
- ↑ David W. Weisrock, Luke J. Harmon, Allan Larson: Resolving Deep Phylogenetic Relationships in Salamanders: Analyzes of Mitochondrial and Nuclear Genomic Data. In: Systematic Biology. 54 (5), 2005, pp. 758-777. doi: 10.1080 / 10635150500234641
- ↑ R. Alexander Pyron, John J. Wiens: A large-scale phylogeny of Amphibia including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians. In: Molecular Phylogenetics and Evolution. 61, 2011, pp. 543-583. doi: 10.1016 / j.ympev.2011.06.012
- ^ Richard Estes, Ilya Darevsky: Fossil amphibians from the Miocene of the North Caucasus, USSR In: Journal of the Paleontological Society of India. vol 20, 1977, pp. 164-169.



