Wolbachia pipientis
| Wolbachia pipientis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
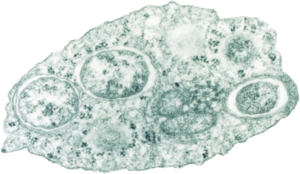
Wolbachia pipientis inside an insect cell |
||||||||||||
| Systematics | ||||||||||||
|
||||||||||||
| Scientific name of the genus | ||||||||||||
| Wolbachia | ||||||||||||
| Hertig, 1936 | ||||||||||||
| Scientific name of the species | ||||||||||||
| Wolbachia pipientis | ||||||||||||
| Hertig , 1936 |
Wolbachia pipientis is a gram-negative bacterium and the only species of the genus Wolbachia in the family Anaplasmataceae . The pleomorphic bacteria can appear as bacilli from 0.5 to 1.3 µm in length, as cocci from 0.25 to 1.0 µm in diameter and as giant forms from 1 to 1.8 µm in diameter. The bacterium has been shown to be sensitive to doxycycline and rifampicin in vitro and in vivo . The bacteria grow obligatorily in the vacuoles of the cells of their hosts or symbionts and cannot be cultivated outside of this environment. However, since 1997 there have been cell lines from eggs of the Asian tiger mosquito ( Aedes albopictus ), with and without infection with Wolbachia pipientis
Wolbachia pipientis was first detected in the gonads of the common mosquito . So far, the bacterium has been identified in insects and other arthropods such as crustaceans and arachnids and in filaria . The relationship between Wolbachia and their hosts or symbionts is varied. It ranges from a pathogenic effect observed in species of Drosophila to parasitism and mutualism to the obligatory symbiosis . Wolbachia influences the reproduction of the hosts in a variety of ways , which overall benefits the female animals and male offspring in the embryonic stage is killed or phenotypically feminized and thus excluded from further reproduction. In the case of insects, the proportion of infected species is estimated at around 16 percent.
Many of the hosts or symbionts involved are vectors of infectious diseases . They include the yellow fever mosquito , the Anopheles gambiae complex, and various ticks . Among the filaria there are important parasites which are pathogenic to humans, such as Onchocerca volvulus , Wuchereria bancrofti and Brugia malayi . Wolbachia has been shown to regulate the pathogen load in some vectors. The possibility of disrupting the reproduction of insect vectors by exposing infected males or of combating pathogenic filariae in the body of the diseased animals or humans by killing their obligate symbionts, Wolbachia , is the subject of more recent research.
features
Wolbachia pipientis is a gram-negative and pleomorphic bacterium. The shapes described are bacilli from 0.5 to 1.3 µm in length, cocci from 0.25 to 1.0 µm in diameter and giant shapes from 1 to 1.8 µm in diameter. The genus Wolbachia differs from the genera Anaplasma , Ehrlichia and Neorickettsia in that they do not form morulae and only colonize arthropods and filariae. It also differs from its sister taxa in its 16S rRNA , on which the bacterial system is based today .
The genome of several strains of Wolbachia pipientis has already been sequenced. The strain wMel (supergroup A, from Drosophila melanogaster ) has 1,267,782 base pairs and 1271 protein-coding genes, while the strain wBm (supergroup D, from Brugia malayi ) has only 1,080,084 base pairs and 806 protein-coding genes. Bacteria, which are obligate symbionts of insects, also have a genome that is smaller than that of related species living in the wild. Examples are Buchnera aphidicola , a symbiote of the pea louse , Blochmannia sp. in horse ants and Wigglesworthia glossinidia in tsetse flies . The smaller genome of the obligate symbionts in Wolbachia is understood as an evolutionary adaptation. Accordingly, the genes still present in parasitic supergroups for mechanisms of penetration into the hosts and their manipulation have been lost as useless.
Way of life
Wolbachia lives obligatorily in the vacuoles of the cells of their hosts or symbionts, in which they reproduce through schizotomy . In arthropods, the Wolbachia are mainly found in the cytoplasm of cells of the sexual organs, but also of nerve cells and blood cells . In filariae, the cells of the lateral nerve cords and the female reproductive organs are colonized. In all hosts, vertical transmission takes place from one generation to the next, with male hosts only being involved indirectly or not at all. The large distribution among the arthropods and other invertebrates results from a horizontal transmission between the hosts that occurs over and over again over long periods of time.
Effect on the hosts
The effects of Wolbachia on their hosts are understood as strategies that ensure a high transmission rate and wide distribution for the bacterium.
Feminization
In the case of the Rollassel Armadillidium vulgare , the hormonal balance of the offspring is disturbed, so that phenotypic females develop from genotypically male individuals . Since cytoplasmic features, mitochondria and also Wolbachia of infected animals are only passed on from females to the next generation, feminization is associated with an advantage for Wolbachia . The mechanism is by suppressing the development of those glands that produce male sex hormones. The feminized genetic males are available as sexual partners to the uninfected males, although the latter, if given the choice, prefer genetic females. In addition, the feminization is often incomplete, the affected individuals show a mixture of male and female characteristics. In feminized populations there is a shortage of males, which is partly compensated for by greater sexual activity of the remaining uninfected males.
The only known insect to date that shows a feminizing effect of Wolbachia is the butterfly Eurema hecabe . The removal of Wolbachia by the administration of an antibiotic means that the affected animals only produce male offspring. Feminization was also suspected in the economically important plant pest Ostrinia scapulalis , a small butterfly; in fact, the exclusively female progeny of infected females is caused by killing the male embryos.
Since the complete elimination of reproductive genetic males in a population would result in the extinction of both the host and its parasite, a number of mechanisms exist for the conservation of the species. When Sow Oniscus asellus the offspring are less than 88 percent of Wolbachia infected. This ensures that the next generation will always have reproductive males. In insect species with male heterogamy , feminization is associated with a high mortality rate among the offspring. Hence, groups like the hymenoptera are not affected by the feminization of the males.
parthenogenesis
Another strategy to favor female individuals as carriers of the Wolbachia is parthenogenesis . Because all offspring are female and are carriers of the infection, the reproductive success of the bacterium is doubled. Parthenogenesis induced by Wolbachia has so far been detected in three orders , the fringed winged (Thysanoptera) and hymenoptera (Hymenoptera) in insects and the trombidiformes in arachnids . Parthenogenesis in Wolbachia-infected arthropods is thus concentrated on species with haplodiploid sex determination.
Among the weevils , a single parthenogenetic species, Naupactus tesselatus , has been identified as the carrier of Wolbachia. However, it is not yet clear whether the bacterium played a role in the development of parthenogenesis in this and other parthenogenetic weevils. The Wolbachia found in some species of the genus Drosophila do not seem to be related to parthenogenesis. Wolbachia induce parthenogenesis in different ways. Three ways have been described, two automictic and one apomictic . In contrast to the strategies of feminization, male-killing and cytoplasmic incompatibility, inducing parthenogenesis can lead to the extinction of the male sex. But there are also cases, as with some species of the wasp genus Trichogramma , in which infected and non-infected individuals coexist. The infected unfertilized females reproduce parthenogenetically and produce only infected female offspring, while the uninfected females produce unfertilized uninfected female offspring and fertilized uninfected males. The preference for female offspring can make males rare. A mutation was found in the wasp Telenomus nawai (Hymenoptera: Scelionidae ), which allows uninfected females to produce large numbers of male offspring, both unfertilized and fertilized.
Male killing
Male Killing (MK) refers to the killing of male offspring during embryogenesis. This strategy can only be beneficial to the Wolbachia if it favors the infected sisters of the dying males. This is regularly the case where siblings compete with one another for scarce resources. It has been observed that females of the tropical butterfly Hypolimnas bolina infected with Wolbachia produce only female offspring. The small butterfly Ostrinia scapulalis is one of the insects in which infection with Wolbachia leads to the death of the male offspring . While male individuals die off as larvae, female animals are inevitably dependent on Wolbachia. If the bacterium is killed with tetracycline , the females die too. In the experiment, infected males could be generated by microinjection of a small number of Wolbachia. Tetracycline treatment of females shortly before oviposition also resulted in the development of viable infected male offspring if infected to a non-fatal extent. The bodies of these males had a mixture of male and female tissues. A higher bacterial load also led to the death of male larvae in these experiments.
Other insects in which the male killing strategy is pursued are the fruit flies Drosophila bifasciata and Drosophila innublia , the beetles Tribolium madens and Adalia bipunctata and the butterfly Acraea encedon . The pseudoscorpion Cordylochernes scorpioides is also one of the species concerned.
Cytoplasmic incompatibility
The Cytoplasmic incompatibility (CI) is the most common effect of an infection with Wolbachia. Mating an infected male with a female that is not infected or with a different strain leads to increased mortality of the offspring. In extreme cases, all offspring die in the embryonic phase. As a result of this mismatch, the affected females are effectively excluded from reproduction; only the carriers of a compatible bacterial strain produce viable infected offspring with infected males. In relation to the total population, infected females are favored over non-infected females in terms of reproductive success.
The described effect was not only predicted theoretically, but could also be observed in the 1980s with the spread of Wolbachia in an originally uninfected California population of Drosophila simulans . The exact mechanism of action is still unknown, the sperm of infected males do not contain Wolbachia. During spermatogenesis, the sperm must have been manipulated in such a way that when the eggs are fertilized, depending on the infection status of the female, they produce viable or non-viable offspring.
The cytoplasmic incompatibility can cause a number of phenotypic changes, which also occur with mutations of the genes maternal haploid , ms (3) K81 and sésame of the fruit fly Drosophila melanogaster, which is often used as a model organism in genetics . An infection with Wolbachia in developmental biology laboratories can also falsify test results when investigating mutations that affect reproduction . A large number of the Drosophila strains used in research are infected with Wolbachia.
Affecting oogenesis
The parasitoid wasp Asobara tabida depends on the presence of Wolbachia pipientis for oogenesis . The mechanism has not yet been studied in detail, but treatment with antibiotics to get rid of the wolbachia will stop the females from producing fertile eggs. This results in the exclusion of uninfected females from reproduction. Asobara tabida is always infected with Wolbachia from three strains, only one influencing oogenesis and the other two causing cytoplasmic incompatibility.
In Vitro Cultivation
Wolbachia pipientis depends on living host cells and cannot be kept alive in a nutrient medium alone. In 1997, however, it was possible to cultivate a cell line naturally infected with Wolbachia pipientis from eggs of the Asian tiger mosquito ( Aedes albopictus ). The nutrient medium was a mixture of equal parts Mitsuhashi Maramorosch Insect Medium and Schneider's Insect Medium , to which 10 to 15 percent fetal calf serum was added. This Wolbachia strain is also called the O'Neill strain after the developer of the method and can also be propagated in a special nutrient medium on cultures of fibroblasts from human embryonic lung tissue and on another Aedes albopictus cell line .
In 2000 a group of Japanese molecular geneticists discovered genetic material of the bacteriophage WO in the chromosomes of different strains of Wolbachia . Like other species-specific bacteriophages, the bacteriophage WO plays a role in the synthesis of a microbial toxin by encoding the genetic material for toxin production.
Hosts and symbiotes
Wolbachia infect a wide range of organisms. Arthropod infections are mostly of a parasitic nature and nematode infections are more of a symbiotic nature. Vertebrates are not infected.
arthropod
Estimates of the proportion of insect species infected by Wolbachia pipientis range from 16 to 76 percent. There are also numerous other species of arthropods and nematodes. The distribution within the taxonomic groups is very different. In the case of animal lice , Wolbachia seem to infect almost all species, while the mosquitoes of the genus Anopheles , which are important vectors of numerous infectious diseases, are free from natural infections.
Among the terrestrial isopods several types with Wolbachia infected supergroup B that cause feminization of male infected animals.
Filaria
Some filariae pathogenic to humans such as Onchocerca volvulus , Wuchereria bancrofti , Brugia malayi and Brugia timori are obligatory carriers of the endosymbiotic Wolbachia. Dirofilaria immitis is a species of veterinary medicine as the causative agent of heartworm disease in dogs. The Wolbachia are passed on to the next generation with the egg cells and, according to phylogenetic studies, have been symbiotically linked to the filariae for millions of years. The joint evolution of both species and molecular genetic studies, which did not reveal any evidence of defense mechanisms against Wolbachia in the hosts and reactions of the Wolbachia to it, support the thesis of the mutualistic relationship.
The spread of Wolbachia among the filariae probably goes back to an infection event that affected the common ancestors of the subfamilies Onchocercinae and Dirofilariinae . It is still unclear how large the proportion of obligate and facultative carriers of Wolbachia is among the species of Filaria, and why obligate carriers and non-carriers occur in closely related species. Apparently, Wolbachia offers wearers an evolutionary advantage that has not yet been identified. In individual species, the symbiotic bond with Wolbachia has been lost again in the course of evolution. Such a loss must have occurred at least six times in the onchocercid phylogeny .
The human diseases caused by these filariae, onchocerciasis of the skin and eyes and lymphatic filariasis with the particularly severe form of elephantiasis , are among the most serious and widespread parasitoses . The pathogens are transmitted by mosquitoes and flies, which ingest microfilariae from an infected host during a blood meal and pass them on to the following hosts. In the ten to fifteen years following a new infection with Onchocerca and five or more years with lymphatic filariasis, the filariae grow in the host, multiply millions of times and can be taken up and spread by bloodsuckers. At the beginning of the 21st century, 200 million people worldwide were infected with filariae and more than a million people were exposed.
In filariae, Wolbachia infects the cells of the lateral nerve cords of both sexes in all stages, from microfilariae to adult animals. In addition, the reproductive organs and egg cells of the female filariae and the precursors of the lateral nerve cords are infected in the embryonic stages, but not the reproductive organs of the male filariae. Presumably in connection with reproduction, Wolbachia multiply in the bodies of the female filaria shortly after the infestation of a mammalian host. The genome of the filarial nematode as Brugia malayi no genes have been identified that in other animals for the production of malice ensure that Wolbachia is attributed to the supply of filarial with hemes.
Wolbachia make a decisive contribution to the development, reproduction and pathogenic effects of the filariae. They cause the accumulation and activation of neutrophilic granulocytes around the filaria and activate macrophages . The neutrophils, in turn, are involved in killing and breaking down the microfilariae and in the development of onchocerciasis as the place of copulation of sexually mature Onchocerca volvulus . In the mouse model of onchocerciasis, wolbachia and the neutrophil reaction they cause make a decisive contribution to keratitis as the most serious symptom of the disease, while the eosinophilic granulocytes that are also involved are activated by the presence of the microfilariae and the macrophages by both.
Wolbachia in biological pest control
The loss of the endosymbiont Wolbachia through the administration of antibiotics, exposure to heat or the withdrawal of certain nutrients has shown a multitude of effects on the host in experiments: incomplete development, deviating coloration of the exoskeleton , short stature, sterility and death.
Aedes aegypti and other mosquito species losetheir ability to reproducewhen infected with Wolbachia pipiens . This can possibly be used in a targeted manner by releasing large numbers of Wolbachia-infected male mosquitoes into the environment and the resulting offspring from healthy female animals and infected male mosquitoes are not viable. The animals do not experience a second reproductive period, so that the population decreases dramatically in size. The mosquito species affected are vectors for various human-pathogenic arboviruses , such as the dengue virus and the malaria pathogen Plasmodium .
The conventional therapy of filariasis consists in long-term therapy with filaricidal agents such as ivermectin , albendazole and diethylcarbamazine , usually in combination. The adult worms are not killed safely, so that the treatment often has to extend over several years until they naturally die. In addition, after the death of the microfilariae, the therapy leads to the release of Wolbachia or lipopolysaccharide- like molecules, which can cause unwanted immune reactions. Killing the Wolbachia through the use of antibiotics such as doxycycline could be a way of effectively fighting filariasis. The loss of Wolbachia leads to long-term or permanent sterility of the filariae ready to reproduce, so that reproduction no longer takes place. In animal experiments, a filaricidal effect was also observed in the treatment of bovine onchocerciasis. However, therapy with doxycycline is contraindicated in pregnant women, breastfeeding women and children under ten years of age, so that conventional filaricides must continue to be used for these patients.
Systematics
Initial description
Wolbachia was discovered in 1924 by the American microbiologists Marshall Hertig and Simeon Burt Wolbach . The first description was in 1936 by Hertig, naming the genus after his colleague Wolbach and leaning the specific epithet on the scientific name of the type host Culex pipiens .
External system
Wolbachia was initially included in the tribe Wolbachieae of the Rickettsiaeceae family. As part of a revision of the Rickettsiales order by the US parasitologist Cornelius Becker Philip , it was placed in the Anaplasmataceae family in 1956 with the genera Aegyptianella , Anaplasma , Ehrlichia and Neorickettsia . This relationship has now been confirmed by molecular genetic studies. Wolbachia occupies a position between the worm- associated bacteria of the genus Neorickettsia and the anaplasma and Ehrlichia transmitted by ticks .
In the second half of the 20th century, several species of the genus Wolbachia were described, which are now in other genera or are only synonyms of Wolbachia pipientis :
- Wolbachia melophagi ( Nöller 1917) Philip 1956 is a synonym of Bartonella melophagi ;
- Wolbachia persica Suitor & Weiss 1961 is a synonym of Francisella persica ;
- Wolbachia popcon is a misspelling of W. popcorn used in the second edition of Bergey's Manual of Systematic Bacteriology ;
- Wolbachia popcorn Min & Benzer 1997 designates only one strain of popcorn due to the appearance of infected cells;
- Wolbachia postica Hsiao & Hsiao 1985 is a synonym of W. pipientis (supergroup B).
Internal system
Although only one species of the genus Wolbachia is recognized today, a high degree of diversity is observed within the species. This diversity is not expressed, as usual, in the form of the description of further species, but through the division into supergroups .
Wolbachia pipientis currently distinguishes 16 supergroups , which are designated with the letters A to Q and form a clade . Supergroup G has proven to be a recombination of supergroups A and B. A description of the supergroup R isolated from cave spiders of the genus Telema was contradicted in 2016. An attempt to split the species Wolbachia pipientis into numerous species, whereby only the supergroup B should remain as Wolbachia pipientis , met with rejection.
Research history
Wolbachia pipientis was discovered in 1924 by the American entomologist Marshall Hertig and the pathologist Simeon Burt Wolbach . At the time, Hertig and Wolbach were researching the vectors that transmit rickettsia in the United States and cause infectious diseases such as typhus and Rocky Mountain spotted fever . One of their discoveries was an initially unnamed rickettsia-like microorganism from the gonads of the common mosquito ( Culex pipiens ). It was not until 1936 that Hertig published the first description of Wolbachia pipientis .
In the decades that followed, Wolbachia was a largely neglected microorganism. In the 1960s and 1970s, several researchers observed unusual structures in the egg cells of various filariae that they could not identify. It was not until 1995 that the perpetrators were recognized as endosymbionts of the genus Wolbachia . With the increased efforts of the World Health Organization to eradicate filariasis , which also included the decoding of the genomes of human-pathogenic filariasis , bacterial DNA was found that was initially interpreted as contamination of the samples. They too were caused by Wolbachia pipientis .
1973 were Armadillidium vulgare first time a country Assel as host of Wolbachia pipientis identified. Other species followed in the years that followed. The infected woodlice are characterized by the fact that male offspring of infected woodlice are feminized.
In 1977 people began to understand that Wolbachia as a rule do not parasitize their hosts, but rather enter into a mutualistic relationship with them . This was concluded from the fact that the killing of Wolbachia in filariae in particular is associated with negative consequences for the hosts up to and including death.
In 2005 the genome of Wolbachia strains of the filariae was deciphered, so that a comparison with the genome of the strains of arthropods was possible.
Web links
Individual evidence
- ↑ a b c d e f g h i j Bernard La Scola , Claudio Bandi , Didier Raoult : Genus IV. Wolbachia Hertig 1936, 472 AL . In: George M. Garrity , Don J. Brenner , Noel R. Krieg, James T. Staley (Eds.): Bergey's Manual of Systematic Bacteriology . Second edition. Volume Two. The Proteobacteria. Part C. The Alpha-, Beta, Delta-, and Epsilonproteobacteria . Springer, New York 2005, ISBN 0-387-24145-0 , pp. 138-143 .
- ↑ a b c d e f Maurizio Casiraghi, Emanuele Ferri, Claudio Bandi: Wolbachia: Evolutionary Significance in Nematodes . In: Achim Hörauf, Ramakrishna U. Rao (Hrsg.): Wolbachia: A Bug's Life in another Bug (= Heinz Zeichenhardt, Brian WJ Mahy [Hrsg.]: Issues in Infectious Diseases . Volume 5 ). Karger, Basel a. a. 2007, ISBN 978-3-8055-8180-6 , pp. 15-30 .
- ↑ Kenneth Pfarr, Jeremy Foster, Barton Slatko: It Takes Two: Lessons From the First Nematode Wolbachia Genome Sequence . In: Achim Hörauf, Ramakrishna U. Rao (Hrsg.): Wolbachia: A Bug's Life in another Bug (= Heinz Zeichenhardt, Brian WJ Mahy [Hrsg.]: Issues in Infectious Diseases . Volume 5 ). Karger, Basel a. a. 2007, ISBN 978-3-8055-8180-6 , pp. 52-65 .
- ^ R. Yamada, JC Brownlie, EA McGraw, SL O'Neill: Insights into Wolbachia Biology Provided through Genomic Analysis . In: Achim Hörauf, Ramakrishna U. Rao (Hrsg.): Wolbachia: A Bug's Life in another Bug (= Heinz Zeichenhardt, Brian WJ Mahy [Hrsg.]: Issues in Infectious Diseases . Volume 5 ). Karger, Basel a. a. 2007, ISBN 978-3-8055-8180-6 , pp. 77-89 .
- ↑ a b c d e f g h i j k l m n o Michael E. Clark: Wolbachia Symbiosis in Arthropods . In: Achim Hörauf, Ramakrishna U. Rao (Hrsg.): Wolbachia: A Bug's Life in another Bug (= Heinz Zeichenhardt, Brian WJ Mahy [Hrsg.]: Issues in Infectious Diseases . Volume 5 ). Karger, Basel a. a. 2007, ISBN 978-3-8055-8180-6 , pp. 90-123 .
- ^ Frédéric Landmann: The Wolbachia Endosymbionts . In: Microbiology Spectrum . tape 7 , no. 2 , 2019, BAI-0018-2019 , doi : 10.1128 / microbiolspec.BAI-0018-2019 .
- ↑ a b Shinji Masui, Satoru Kamoda, Tetsuhiko Sasaki, Hajime Ishikawa: Distribution and Evolution of Bacteriophage WO in Wolbachia, the Endosymbiont Causing Sexual Alterations in Arthropods . In: Journal of Molecular Evolution . tape 51 , 2000, pp. 491-497 , doi : 10.1007 / s002390010112 .
- ↑ Franck Dedeine, Fabrice Vavre, Frédéric Fleury, Benjamin Loppin, Michael E. Hochberg, Michel Boulétreau: Removing symbiotic Wolbachia bacteria specifically inhibits oogenesis in a parasitic wasp . In: Proceedings of the National Academy of Sciences of the United States of America . tape 98 , no. 11 , 2001, p. 6247-6252 , doi : 10.1073 / pnas.101304298 .
- ↑ Scott L. O'Neill, MM Pettigrew, SP Sinkins, HR Braig, TG Andreadis, RB Tesh: In vitro cultivation of Wolbachia pipientis in an Aedes albopictus cell line . In: Insect Molecular Biology . tape 6 , no. 1 , 1997, p. 33-39 , doi : 10.1046 / j.1365-2583.1997.00157.x .
- ↑ a b c d e f Wieslaw J. Kozek, Ramakrishna U. Rao: The Discovery of Wolbachia in Arthropods and Nematodes - A Historical Perspective . In: Achim Hörauf, Ramakrishna U. Rao (Hrsg.): Wolbachia: A Bug's Life in another Bug (= Heinz Zeichenhardt, Brian WJ Mahy [Hrsg.]: Issues in Infectious Diseases . Volume 5 ). Karger, Basel a. a. 2007, ISBN 978-3-8055-8180-6 , pp. 1-14 .
- ↑ a b c d Achim Hörauf , Sabine Mand, Dietrich W. Büttner: Doxycycline for chemotherapy of filariasis: Elimination of Wolbachia, essential bacterial endosymbionts in worms . In: Deutsches Ärzteblatt . tape 100 , no. 37 . Deutscher Ärzte-Verlag , September 12, 2003, p. A2383-A2386 ( aerzteblatt.de ).
- ↑ Laura Kramer, John W. McCall, Giulio Grandi, Claudio Genchi: Wolbachia and Its Importance in Veterinary Filariasis . In: Achim Hörauf, Ramakrishna U. Rao (Hrsg.): Wolbachia: A Bug's Life in another Bug (= Heinz Zeichenhardt, Brian WJ Mahy [Hrsg.]: Issues in Infectious Diseases . Volume 5 ). Karger, Basel a. a. 2007, ISBN 978-3-8055-8180-6 , pp. 124-132 .
- ↑ Katelyn Fenn, Mark Blaxter: Coexist, Cooperate and Thrive: Wolbachia as Long-Term Symbionts of Filarial Nematodes . In: Achim Hörauf, Ramakrishna U. Rao (Hrsg.): Wolbachia: A Bug's Life in another Bug (= Heinz Zeichenhardt, Brian WJ Mahy [Hrsg.]: Issues in Infectious Diseases . Volume 5 ). Karger, Basel a. a. 2007, ISBN 978-3-8055-8180-6 , pp. 66-76 .
- ^ A b Achim Hörauf, Kenneth Pfarr: Wolbachia Endosymbionts: An Achilles' Heel of Filarial Nematodes . In: Achim Hörauf, Ramakrishna U. Rao (Hrsg.): Wolbachia: A Bug's Life in another Bug (= Heinz Zeichenhardt, Brian WJ Mahy [Hrsg.]: Issues in Infectious Diseases . Volume 5 ). Karger, Basel a. a. 2007, ISBN 978-3-8055-8180-6 , pp. 31-51 .
- ↑ Amélie v. Saint André, Nathan M. Blackwell, Laurie R. Hall, Achim Hörauf, Norbert W. Brattig, Lars Volkmann, Mark J. Taylor, Louise Ford, Amy G. Hise, Jonathan H. Lass, Eugenia Diaconu, Eric Pearlman: The Role of Endosymbiotic Wolbachia Bacteria in the Pathogenesis of River Blindness . In: Science . tape 295 , no. 5561 , 2002, pp. 1892–1895 , doi : 10.1126 / science.1068732 .
- ^ Ilaria Dorigatti, Clare McCormack, Gemma Nedjati-Gilani, Neil M. Ferguson: Using Wolbachia for dengue control: insights from modeling . In: Trends in parasitology . tape 34 , no. 2 , February 2018, ISSN 1471-4922 , p. 102–113 , doi : 10.1016 / j.pt.2017.11.002 , PMID 29183717 , PMC 5807169 (free full text).
- ^ Margie Mason: Wolbachia bacteria: Researchers infect mosquitoes with dengue inhibitors . November 6, 2013 ( welt.de [accessed July 20, 2019]).
- ^ Fabio M. Gomes, Carolina Barillas-Mury : Infection of anopheline mosquitoes with Wolbachia: Implications for malaria control . In: PLoS Pathogens . tape 14 , no. 11 , November 15, 2018, ISSN 1553-7366 , doi : 10.1371 / journal.ppat.1007333 , PMID 30440032 , PMC 6237385 (free full text).
- ↑ Fight against tropical diseases: With bacteria against malaria mosquitoes . In: Spiegel Online . May 31, 2016 ( spiegel.de [accessed July 20, 2019]).
- ↑ - Bacteria protect against malaria. Retrieved on July 20, 2019 (German).
- ↑ Helen F. Cross, Melang Haarbrink, Gill Egerton, Maria Yazdanbakhsh, Mark J. Taylor: Severe reactions to filarial chemotherapy and release of Wolbachia endosymbionts into blood . In: The Lancet . tape 358 , no. 9296 , 2001, p. 1873-1875 , doi : 10.1016 / S0140-6736 (01) 06899-4 .
- Jump up ↑ Paul B. Keizer, Stacey M. Reynolds, Kwablah Awadzi, Eric A. Ottesen, Mark J. Taylor, Thomas B. Nutman: Bacterial Endosymbionts of Onchocerca volvulus in the Pathogenesis of Posttreatment Reactions . In: The Journal of Infectious Diseases . tape 185 , no. 6 , 2002, pp. 805-811 , doi : 10.1086 / 339344 .
- ^ A b Marshall Hertig , S. Burt Wolbach : Studies on rickettsia-like microorganisms in insects . In: Journal of medical research . tape 44 , no. 3 , 1924, pp. 329–374, panels XXVII-XXX , PMC 2041761 (free full text).
- ^ A b Marshall Hertig: The Rickettsia, Wolbachia pipientis (gen. Et sp.n.) and Associated Inclusions of the Mosquito, Culex pipiens . In: Parasitology . tape 28 , no. 4 , 1936, pp. 453-486 , doi : 10.1017 / S0031182000022666 .
- ↑ a b Cornelius B. Philip: Comments on the classification of the order Rickettsiales . In: Canadian Journal of Microbiology . tape 2 , no. 3 , 1956, pp. 261-270 , doi : 10.1139 / m56-030 .
- ^ Wilhelm Nöller : Blood and Insect Flagellates. Growing on plates . In: Archives for ship and tropical hygiene . tape 21 , 1917, pp. 53-94 .
- ↑ J. Stephen Dumler, Anthony F. Barbet, Cornelis PJ Bekker, Gregory A. Dasch, Guy H. Palmer, Stuart C. Ray, Yasuko Rikihisa, Fred R. Rurangirwa: Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and 'HGE agent' as subjective synonyms of Ehrlichia phagocytophila . In: International Journal of Systematic and Evolutionary Microbiology . tape 51 , 2001, p. 2145-2165 , doi : 10.1099 / 00207713-51-6-2145 .
- ^ Earl C. Suitor, Emilio Weiss: Isolation of a Rickettsialike Microorganism (Wolbachia persica, n. Sp.) From Argas persicus (Oken) . In: The Journal of Infectious Diseases . tape 108 , no. 1 , 1961, pp. 95-106 , JSTOR : 30099293 .
- ↑ Mark L. Niebylski, Mort G. Peacock, Elizabeth R. Fischer, Stephen F. Porcella, Tom G. Schwan: Characterization of an Endosymbiont Infecting Wood Ticks, Dermacentor andersoni, as a Member of the Genus Francisella . In: Applied and Environmental Microbiology . tape 63 , no. 10 , 1997, pp. 3933-3940 , PMC 168705 (free full text).
- ↑ a b Kyung-Tai Min, Seymour Benzer : Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death . In: Proceedings of the National Academy of Sciences of the United States of America . tape 94 , no. 20 , 1997, pp. 10792-10796 , doi : 10.1073 / pnas.94.20.10792 .
- ↑ Catherine Hsiao, TH Hsiao: Rickettsia as the cause of cytoplasmic incompatibility in the alfalfa weevil, Hypera postica . In: Journal of Invertebrate Pathology . tape 45 , no. 2 , 1985, pp. 244-246 , doi : 10.1016 / 0022-2011 (85) 90016-3 .
- ↑ Ehsan Sanaei, Martin Husemann, Marjan Seiedy, Michael Rethwisch, Midori Tuda, Teodora B. Toshova, Min Jee Kim, Daniela Atanasova, Iksoo Kim: Global genetic diversity, lineage distribution, and Wolbachia infection of the alfalfa weevil Hypera postica (Coleoptera: Curculionidae) . In: Ecology and Evolution . tape 9 , no. 17 , 2019, pp. 9546-9563 , doi : 10.1002 / ece3.5474 .
- ↑ Yury Ilinsky, Oleg E. Kosterin: Molecular diversity of Wolbachia in Lepidoptera: Prevalent allelic content and high recombination of MLST genes . In: Molecular Phylogenetics and Evolution . tape 109 , 2017, p. 164–169 , doi : 10.1016 / j.ympev.2016.12.034 .
- ↑ Guan-Hong Wang, Ling-Yi Jia, Jin-Hua Xiao, Da-Wei Huang: Discovery of a new Wolbachia supergroup in cave spider species and the lateral transfer of phage WO among distant hosts . In: Infection, Genetics and Evolution . tape 41 , 2016, p. 1–7 , doi : 10.1016 / j.meegid.2016.03.015 .
- ↑ Michael Gerth: Classification of Wolbachia (Alphaproteobacteria, Rickettsiales): No evidence for a distinct supergroup in cave spiders . In: Infection, Genetics and Evolution . tape 43 , 2016, p. 378-380 , doi : 10.1016 / j.meegid.2016.05.034 .
- ↑ Shamayim T. Ramírez-Puebla, Luis E. Servín-Garcidueñas, Ernesto Ormeño-Orrillo, Arturo Vera-Ponce de León, Mónica Rosenblueth, Luis Delaye, Julio Martínez, Esperanza Martínez-Romero: Species in Wolbachia? Proposal for the designation of 'Candidatus Wolbachia bourtzisii', 'Candidatus Wolbachia onchocercicola', 'Candidatus Wolbachia blaxteri', 'Candidatus Wolbachia brugii', 'Candidatus Wolbachia taylori', 'Candidatus Wolbachia collembolicola' for the different species within Wolbachia supergroups . In: Systematic and Applied Microbiology . tape 38 , no. 6 , 2015, p. 390-399 , doi : 10.1016 / j.syapm.2015.05.005 .
- ↑ Amelia RI Lindsey, Seth R. Bordenstein, Irene LG Newton, Jason L. Rasgon: Species in Wolbachia? Proposal for the designation of 'Candidatus Wolbachia bourtzisii', 'Candidatus Wolbachia onchocercicola', 'Candidatus Wolbachia blaxteri', 'Candidatus Wolbachia brugii', 'Candidatus Wolbachia taylori', 'Candidatus Wolbachia collembolicola' for the different species within Wolbachia supergroups . In: Systematic and Applied Microbiology . tape 38 , no. 6 , 2015, p. 390-399 , doi : 10.1016 / j.syapm.2015.05.005 .
- ↑ Shamayim T. Ramírez-Puebla, Luis E. Servín-Garcidueñas, Ernesto Ormeño-Orrillo, Arturo Vera-Ponce de León, Mónica Rosenblueth, Luis Delaye, Julio Martínez, Esperanza Martínez-Romero: A response to Lindsey et al. "Wolbachia pipientis should not be split into multiple species: A response to Ramírez-Puebla et al." In: Systematic and Applied Microbiology . tape 39 , no. 3 , 2016, p. 223-225 , doi : 10.1016 / j.syapm.2016.03.004 .
- ↑ Laura Baldo et al .: Wolbachia are present in southern african scorpions and cluster with supergroup F. In: Current Microbiology 2007, Volume 55, pp. 367-373, doi : 10.1007 / s00284-007-9009-4 .
- ↑ Ramakrishna U. Rao, Achim Hörauf: Foreword . In: Achim Hörauf, Ramakrishna U. Rao (Hrsg.): Wolbachia: A Bug's Life in another Bug (= Heinz Zeichenhardt, Brian WJ Mahy [Hrsg.]: Issues in Infectious Diseases . Volume 5 ). Karger, Basel a. a. 2007, ISBN 978-3-8055-8180-6 , pp. VII-VIII .