Acremonium strictum
| Acremonium strictum | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|

Culture of Acremonium falciforme |
||||||||||||
| Systematics | ||||||||||||
|
||||||||||||
| Scientific name | ||||||||||||
| Acremonium strictum | ||||||||||||
| W. Gams (1971) |
Acremonium strictum is a fungus from the Bionectriaceaen family. The saprotrophic species is found in different environmental conditions in the soil and in dead plant and pizzas. Isolates of the species have been collected in North and Central America, Asia, Europe and Egypt. A. strictum is a causative agent of hyalohyphomycosis and has been identified as an increasingly common pathogen in patients with suppressed immune functions, causing local, dispersed and invasive infections. Although this is extremely rare, A. strictum can also affect individuals with healthy immune systems and newborns. Because of the increasing number of infections caused by A. strictum in the past few years, the need for new medical techniques for identification as well as treatment options has increased considerably.
There is evidence of the involvement of A. strictum in some mycoparasitic relationships; In addition, a wide spectrum of endophytic and parasitic relationships with other organisms has been demonstrated; Further studies are required to determine the use of A. strictum as a reagent for biological pest control and its role in reducing yields. A. strictum involves many products in the metabolism, suggesting its future importance in agriculture and pharmacy.
description
Acremonium is a large polyphyletic genus with about 150 species, many of which are derived from closely related families of the class Sordariomycetes . The genus contains many slow growing, simply structured, anamorphic filamentous fungi that are typically found in moist, cellulosic materials under constantly moist conditions. Characteristic for the morphological features of this genus are hyphae with a septum , which form thin, pointed phialides , which in turn are unicellular, or weakly branched conidiophores . Infections in humans, although rare, occur in people with severely compromised immune systems. A. strictum is mostly found in mycoparasitic relationships and as a plant parasite and endophyte .
Morphological identification
Appearance of the colonies
Acremonium strictum is growing rapidly at 30 ° C on glucose peptone - agar , the mycelium within seven days of no more than 50 mm is growing. The colonies are flat, with a soft, moist, velvety, or flaky texture that sometimes resembles cotton bumps. The color of the mycelia ranges from light pink to orange; sometimes they are yellow, white, or green. The filaments of A. strictum are sometimes connected into stronger structures by multiple layers of cells. The conidia grow as moist lumps or dry chains, and the granular structures produced are white to pale yellow, soft and of variable shape. Subcultures of the fungus can also grow within seven days; the soft, moist, pink mycelia resemble thin cotton.
Microscopic appearance
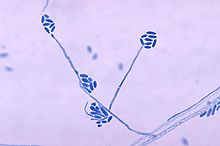
Under the microscope, A. strictum shows long, slender phialides at 30 ° C. The conidia are cylindrical or ellipsoidal and shaped into slimy bundles at the tips of the phialides. At low magnification, pinhead-shaped spore ball formations can be seen.
The species of the genus Acremonium are morphologically very similar, which makes identification difficult. The picture shows the microscopic image of A. falciforme , which shows the morphological similarities to A. strictum . Often several different are Acremonium TYPES simply as Acremonium referred to what the frequency of accurate information on the clinical appearance of A. strictum reduced. Isolates of phylogenetically distantly related Acremonium species show considerable analogies . Since it is also a human pathogen, the diagnosis is made by isolates; H. the determination of the fungus is made from granules in the tissue as well as from the presence of the hyphae upon microscopic examination of biopsies and effluents.
Morphologically similar genera to Acremonium are u. a. Fusarium , Phaeoacremonium , Verticillium , Phialemonium and Lecanicillium .
Genetic identification
The identification of isolates of A. strictum has shown that the fungus is phenotypically diverse and can vary genetically. Due to the phylogenetic ambiguity, an unknown proportion of the literature on A. strictum is based on studies of Acremonium sclerotigenum . The fungus can generally be identified by ITS nuclear region sequence analysis. The analyzes of the large ribosomal subunit ( English ribosomal large subunit , LSU) and the entire small subunit ( English small subunit , SSU) also helps to elucidate the phylogenetic relationships, since these genes are more conserved and are therefore less subject to evolutionary changes. The A. strictum species is divided into three genetic groups. Gene group I is represented by the type of strain CBS 346.70, gene group II by UW836 and gene group III by UWFP940. These gene groups were created on the basis of GenBank data on A. strictum .
Taxonomy
The following synonyms are known:
- Cephalosporium acremonium Corda (1839)
- Cephalosporium acremonium var. Majus Penz. (1882)
- Cephalosporium acremonium var. Uniseptatum Massee ex Sacc. (1892)
- Cephalosporium acremonium var. Natricis Fragner (1958)
- Cephalosporium majus (Penz.) Mussat (1901)
- Haplotrichum acremonium (Corda) Pound & Clem. (1896)
- Hyalopus acremonium (Corda) MAJBarbosa (1941)
- Sarocladium strictum (Gams) Summerbell (2011)
- Tilachlidium medietatis Novobr. (1972)
Infections in humans
Pathophysiology
Acremonium strictum infections in humans are very rare and usually develop after traumatic inoculation of the fungus. Hyalohyphomycosis can occur in people with a weakened immune system and can be recognized by hyaline or colorless hyphae in the infected tissue. Peritonitis and pleurisy as a result of A. strictum infections have also been reported, but skin or subcutaneous infections are rarely reported.
- Most infections in humans have been observed in people with immunodeficiency and have been described as local or disseminated infections of the blood or eye, or as a mycetoma , which often ends up being severe. A. strictum can cause invasive infections such as pneumonia , arthritis , osteomyelitis , endocarditis , meningitis or sepsis in patients with a weakened immune system.
- Infections in immunocompetent individuals usually follow inoculation during injury to the limb or cornea and result in local infections. The fungus can also cause nail fungus , ontomycosis (infection of one or both ears), or infection of burn wounds. Patients with heart valve prostheses infected with A. strictum in the region of the valves can be affected by severe inflammation that can lead to sepsis or multiple organ failure.
- Infections can occur in newborns , albeit rarely, and can have serious consequences.
Many environmental factors such as the frequency of fungi in the soil, rainfall, temperature, humidity and type of vegetation influence the likelihood of a hyalohyphomycosis infection by A. strictum in close contact . Frequent contact with contaminated water combined with high temperatures and humidity increases the risk of infection.
Clinical appearance and treatment
The clinical presentation of infection is poorly defined, but most patients will experience rashes and flu-like symptoms such as high temperature and fatigue. In severe infections, such as in immunocompromised patients, peritonitis and pleurisy, which eventually lead to multiple organ failure, will also occur. In the case of invasive infections, surgery may be required to remove the fungus from the tissues of the body. Due to the limited disease-defined cases and the variances of the clinical appearance as well as due to the uncertain identification of the fungus species, no optimal treatment is available. A. strictum and other Acremonium species are generally resistant to most antifungal drugs ; however, an antifungal susceptibility test is recommended to determine the most appropriate treatment for the particular strain of A. strictum causing the infection. Amphotericin B therapy in conjunction with ketoconazole is usually recommended as the best available treatment.
Biological control
It has been shown that seedlings infected with A. strictum have a high mortality rate. It would be agriculturally important to find biological pesticides for this fungus. The above-ground parts of camel grass ( Cymbopogon schoenanthus ) Hyptis spicigera , the Wandelröschens ( Lantana camara ) and Ocimum americanum were collected and dried for four days. After drying, essential oils were extracted. A number of seeds have been inoculated with fungi, including cohorts of A. strictum . The oils were applied to the infected seeds. After the seeds germinated, a significantly inhibited growth of the A. strictum mycelia was observed.
Interactions with other mushrooms
Acremonium strictum is commonly known as a fungal parasite, which is also reflected in its antagonistic relationship to the silver scab ( Helminthosporium solani ). Silver scab is a potato pathogen that has caused extreme and widespread losses in all potato market classes since it first appeared in the United States. The fungus causes blemishes that reduce the quality of the crops to such an extent that they can no longer be marketed. In more severe cases, the silver scab causes weight loss and lesions in the periderm that give access to other pathogens. In pure culture, the silver scab shows white sectors and rings, a differentiated coloration and reduced sporulation . After infection with A. strictum , these cultures turn uniformly black with no white sectors or rings. A. strictum was able to significantly reduce sporulation of silver scab by 30%, spore germination by 20% and mycelial growth by 8%. This suggests that A. strictum can be used as a biological pest control against the silver scab, which would greatly increase the yield of potato crops.
Interactions with plants
Stem rot
Acremonium strictum is a pathogen to many monocots and dicotyledonous crops, causing desiccation of leaves on one side of the midrib , wilting, and abnormal, discolored conductive tissues of the stem near the base. The conductive tissues form orange, red and brown bundles; the plant eventually dies. The infection with A. strictum is systemic, so that the fungus can be isolated from all plant tissues. The isolates were also found in seeds, which may be the starting point of infection. Crops affected include acacia , alder , fig , bean , cotton , wheat, and corn . Because of its ubiquitous presence in the soil, A. strictum negatively affects many agricultural species, although some research is still needed to elucidate the parasitic interactions and develop strategies for biological pest control.
Root gall nails
The Southern root- ( Meloidogyne incognita ) is a polyphagous nematode ( Nematoda ), the tomato plants can seriously damage by causing lesions on the roots by use of a stylet, which ground-living fungal parasites allows the penetration into the host, and may trigger complex diseases. A. strictum is described as an egg parasite of the southern root knot because the nematode eggs were found empty in infected plants after treatment with A. strictum . The use of A. strictum together with Trichoderma harzianum turned out to be a promising method of combating the root knot in tomato plants.
Strawberries
A. strictum has a complicated relationship with garden strawberries ( Fragaria × ananassa ) in which the fungus can cause lesions and small necrotic light brown spots on the leaves and petioles that multiply as the disease progresses, which is unfavorable to cultivation. Eventually, the necrotic regions expand so that the plant begins to wither, even if crown rot has not been observed at any point in the disease process. Although it appears to be a parasitic relationship, the fungus produces an Inductor Protein (AsES) which provides systemic protection against anthracnosis in the strawberry, which would indicate a symbiotic relationship between the fungus and the plant.
Cangzhu
Cangzhu ( Atractylodes lancea ) is a medicinal plant that grows in central China. A. strictum grows in it as a fungal endophyte and interacts with Cangzhu under arid conditions, giving the plant a moderate tolerance to drought. Under these moderately dry conditions, A. strictum improves the enzyme activity of soluble sugars, proteins and proline as well as antioxidants in the leaf, which reduce the degree of cell membrane oxidation. In Cangzhu, this increases the abscisic acid level and the root-to-shoot ratio. While A. strictum alleviates the effects of low to moderate drought, there is no effect with adequate water supply or severe drought.
Mulberry trees
In Maclura cochinchinensis , Acremonium strictum grows as an endophytic fungus that primarily infects the leaves of the plant. In this connection, A. strictum plays the role of triggering a protective reaction to herbivorous insects.
Natural metabolic products
Acremostrictin
Acremostrictin can be isolated from various strains of A. strictum . It is a highly oxidized, tricyclic lactone - metabolites . This compound gives the plant weak antibacterial properties against Micrococcus luteus , Salmonella enterica subsp. enterica and Proteus vulgaris . However, it has no effect on Bacillus subtilis , Staphylococcus aureus and Escherichia coli . Acremostrictin has been shown to have a concentration-dependent antioxidant activity, which offers protection against cell death induced by oxidative stress . In addition, the inhibition of H 2 O 2 -induced death of human keratinocytic HaCaT cells was demonstrated. When extracted and isolated by filtration, acremostrictin shows up as a colorless crystalline solid.
AsES
The protein AsES ( A. strictum elicitor subtilisin ) is an extracellular inducer that is produced by A. strictum and offers complete systemic protection against anthracnose, which is caused by fungus species of the genus Glomerella in strawberries as well as in "weeds" arable cress ( Arabidopsis thaliana ). Anthracnose can affect all types of tissue and appears as an irregular black spot on the leaf, bleaching of flowers, and fruit and crown rot, causing serious losses in plant and fruit production. ASES has a proteolytic that in an enhancement of activity, which seems to induce an immune response in these types of oxygen radicals and the expression of included in the defense genes such as PR1 ( English Pathogenesis-related protein 1) and Chi2-1 seems to end. Because it also provides systemic protection in plants other than crops, AsES could be included in a possible strategy for combating anthracnosis in plants.
Cephalosporins
A. strictum produces some varieties of cephalosporins , a group of antibiotics.
Industrial use
BMOs
Biogenic manganese oxides ( English Biogenic Mn oxides BMOs) are naturally occurring manganese oxides that have the ability to oxidize various redox- sensitive elements. A. strictum is a Mn (II) oxidizing fungus that forms BMOs through the activity of an Mn (II) oxidase . In the presence of BMOs in buffer solutions without additional nutrients, A. strictum is able to secrete high concentrations of Mn (II) over at least eight days, in which the amount of dissolved manganese (II) increases rapidly within a few hours and is converted to oxidized Mn (II) becomes. An oxygen release of the buffer solution with nitrogen leads to a suppressed Mn (II) conversion; this can be reversed by aeration, from which it can be concluded that dissolved oxygen is required for the Mn (II) secretion and the oxidation process. Adding sodium azide , a poisonous substance, also lowers the rate of excretion of fungal BMOs. The addition of heat showed no change in the conformation of the Mn (II) oxidase in the BMOs below 85 ° C. Freezing the fungal BMOs at −80 ° C for four weeks did not affect the ability to secrete Mn (II) and the reducible manganese continued to dominate the solution. All of this makes fungal BMOs effective sources of Mn (II) when needed. This can be used, for example, to purify water contaminated with Mn (II) without adding any additives except dissolved oxygen. The product is an oxidic phase of Mn (II), which offers additional affinity for other toxic elements and so could be developed into an effective water purification method. Enzymatically active fungal BMOs can be obtained under certain culture conditions and remain active even under conditions that are unfavorable for fungal growth.
Ginsenoside analogs
The fermentation of ginsenoside Rb (1) by A. strictum generates three new compounds:
- 12β-Hydroxydammar-3-one-20 (S) -O-β-D-glucopyranoside,
- 12β, 25-dihydroxydammar- (E) -20 (22) -ene-3-O-β-D-glucopyranosyl- (1 → 2) -β-D-glucopyranoside and
- 12β, 20 (R), 25-trihydroxydammar-3-O-β-D-glucopyranosyl- (1 → 2) -β-D-glucopyranoside.
There are also five known connections:
- Ginsenoside Rd,
- Gypenoside XVII,
- Ginsenoside Rg,
- Ginsenoside F and
- Compound K.
Many of these compounds are metabolites of ginsenoside Rb (1) in mammals, suggesting that fermentation of ginsenoside Rb (1) in A. strictum is similar to that in mammals and a useful reagent for the generation of specific metabolites or related ginsenoside analogs which could later be used for structural elucidation and pharmaceutical research.
Individual evidence
- ↑ a b c d e f g h J. Guarro, W-Gams, I. Puhhol, J. Gene: Acremonium species: new emerging fungal opportunists — in vitro antifungal susceptibilities and review . In: Clinical Infectious Diseases . 25, No. 5, 1997, pp. 1222-1229. doi : 10.1086 / 516098 .
- ↑ a b c d e f W. A. Schell, JR Perfect: Fatal, disseminated Acremonium strictum infection in a neutropenic host . In: Journal of Clinical Microbiology . 34, No. 5, 1996, pp. 1333-1336.
- ↑ a b c d e f g h i j k l m Ajanta Sharma, NK Hazarika, Purnima Barua, MR Shivaprakash, Arunalok Chakrabartie: Acremonium strictum : report of a rare emerging agent of cutaneous hyalohyphomycosis with review of literatures . In: Mycopathologia . 176, No. 5-6, 2013, pp. 435-441. doi : 10.1007 / s11046-013-9709-1 .
- ↑ a b c d e f R. M. Fincher, JF Fisher, RD Lovell, CL Newman, A. Espinel-Ingroff, HJ Shadomy: Infection due to the fungus Acremonium (cephalosporium) . In: Medicine . 70, No. 6, 1991, pp. 398-409. doi : 10.1097 / 00005792-199111000-00005 .
- ↑ a b c d e f g h i H. Perdomo, DA Sutton, D. Garcia, AW Fothergill, J. Cano, J. Gene, RC Summerbell, MG Rinaldi, J. Guarro: Spectrum of clinically relevant Acremonium species in the United States . In: Journal of Clinical Microbiology . 49, No. 1, 2010, pp. 243-256. doi : 10.1128 / jcm.00793-10 .
- ↑ a b c V. V. Rivera-Varas, TA Freeman, NC Gudmestad, GA Secor: Mycoparasitism of Helminthosporium solani by Acremonium strictum . In: Phytopathology . 97, No. 10, 2007, pp. 1331-1337. doi : 10.1094 / phyto-97-10-1331 .
- ↑ a b c d N. R. Chalfoun, CF Grellet-Bournonville, MG Martinez-Zamora, A. Diaz-Perales, AP Castagnaro, JC Diaz-Ricci: Purification and characterization of AsES protein: a subtilisin secreted by Acremonium strictum is a novel plant defense elicitor . In: Journal of Biological Chemistry . 288, No. 20, 2013, pp. 14098-14113. doi : 10.1074 / jbc.m112.429423 .
- ↑ a b T. Yang, S. Ma, CC Dai: Drought degree constrains the beneficial effects of a fungal endophyte on Atractylodes lancea . In: Journal of Applied Microbiology . 117, No. 5, 2014, pp. 1435–1449. doi : 10.1111 / jam.12615 .
- ↑ a b Sheng-Liang Zhou, Shu-Zhen Yan, Qi-Sha Liu, Shuang-Lin Chen: Diversity of endophytic fungi associated with the foliar tissue of a hemi-parasitic plant Macrosolen cochinchinensis . In: Current Microbiology . 70, No. 1, 2014, pp. 58-66. doi : 10.1007 / s00284-014-0680-y .
- ↑ a b G. T. Chen, M. Yang, Y. Song, JQ Zhang, HL Huang, LJ Wu, DA Guo: Microbial transformation of ginsenoside Rb 1 by Acremonium strictum . In: Applied Microbiology and Biotechnology . 77, No. 6, 2008, pp. 1345-1350. doi : 10.1007 / s00253-007-1258-4 .
- ↑ a b Jianing Chang, Yukinori Tani, Hirotaka Naitou, Haruhiko Seyama: Fungal Mn oxides supporting Mn (II) oxidase activity as effective Mn (II) sequestering materials . In: Environmental Technology . 34, No. 19, 2013, pp. 2781-2787. doi : 10.1080 / 09593330.2013.790066 .
- ↑ a b c d e f R. C. Summerbell, C. Gueidan, H.-J. Schroers, GS de Hoog, M. Starink, Y. Arocha Rosete, J. Guarro, JA James: Acremonium phylogenetic overview and revision of Gliomastix , Sarocladium , and Trichothecium . In: Studies in Mycology . 68, 2011, pp. 139-162. doi : 10.3114 / sim.2011.68.06 .
- ↑ a b J. F. Leslie: Sorghum and Millets Diseases . John Wiley & Sons, 2008, ISBN 978-0470384701 , pp. 188-189.
- ↑ a b Jaideep Goswami, Rajesh Kumar Pandey, JP Tewari, BK Goswami: Management of root knot nematode of tomato through application of fungal antagonists, Acremonium strictum and Trichoderma harzianum . In: Journal of Environmental Science and Health . 43, No. 3, 2008, pp. 237-240. doi : 10.1080 / 03601230701771164 .
- ↑ a b c C. K. Campbell, EM Johnson: Identification of Pathogenic Fungi . John Wiley & Sons, 2013, ISBN 978-1118520048 , pp. 178-179.
- ↑ a b T. J. Novicki, K. LaFe, L. Bui, R. Geise, K. Marr, BT Cookson: Genetic diversity among clinical isolates of Acremonium strictum determined during an investigation of a fatal mycosis . In: Journal of Clinical Microbiology . 41, No. 6, 2003, pp. 2623-2328. doi : 10.1128 / jcm.41.6.2623-2628.2003 .
- ↑ a b J. Guarro, A. Del Palacio, J. Cano, CG Gonzalez: A case of colonization of a prosthetic mitrial valve by Acremonium strictum . In: Revista Iberoamericana de Micología . 26, No. 2, 2009, pp. 146-148. doi : 10.1016 / s1130-1406 (09) 70025-7 .
- ↑ a b c d e f g h Species Fungorum . In: Index Fungorum . Retrieved September 3, 2019.
- ↑ a b Acremonium strictum . In: MycoBank . Retrieved September 3, 2019.
- ↑ a b c A. G. Sener, M. Yucesoy, Seckin Senturkun, Ilhan Afsar, SG Yurtsever, M. Turk: A case of Acremonium strictum peritonitis . In: Med. Mycol. . 46, No. 5, 2008, pp. 495-497. doi : 10.1080 / 13693780701851729 .
- ↑ a b A. Koc, M. Sariguzel, T. Artis: Pleuritis caused by Acremonium strictum in a patient with colon adenocarcinoma . In: Mycoses . 51, No. 6, 2008, pp. 554-556. doi : 10.1111 / j.1439-0507.2008.01517.x .
- ↑ PE Zida, P. Sereme, V. Leth, P. Sankara, I. Somda, A. Neva: Importance of seed-borne fungi of sorghum and pearl millet in Burkina Faso and their control using plant extracts . In: Pakistan Journal of Biological Sciences . 11, No. 3, 2008, pp. 321-331. doi : 10.3923 / pjbs.2008.321.331 .
- ↑ a b J. Racedo, SM Salazar, P. Castagnaro, JC Diaz Ricci: A strawberry disease Caused by Acremonium strictum . In: European Journal of Plant Pathology . 137, No. 4, 2013, pp. 649-654. doi : 10.1007 / s10658-013-0279-3 .
- ↑ a b c Elin Julianti, Hana Oh, Kyoung Hwa Jang, Jae Kyun Lee, Sang Kook Lee, Dong-Chang Oh, Ki-Bong Oh, Jongheon Shin: Acremostrictin, a highly oxygenated metabolite from the marine fungus Acremonium strictum . In: Journal of Natural Products . 74, No. 12, 2011, pp. 2592-2594. doi : 10.1021 / np200707y .
- ↑ UniProtKB - R4IR27 (ASES_SARSR) . In: UniProt . Retrieved September 3, 2019.