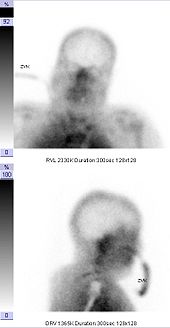
Brain perfusion scintigraphy
The Hirnperfusionsszintigrafie (from latin perfundere flow through, scintillating Funke and Greek γράφειν , Graphein drawing, describe) is a nuclear medicine procedures for two-dimensional and three-dimensional pictorial representation of the regional cerebral blood flow (engl. Regional cerebral blood flow , rCBF).
The cerebral (brain-related) blood flow is increased, decreased or abolished in various diseases in parts of the brain (regional) or in the entire brain (global). The tracers used are 99m Tc -labeled substances that are highly lipophilic , accumulate quickly in the brain and remain there for a long time, in particular hexamethylpropyleneaminooxime (HMPAO) and 99m Tc- ethylcysteinate dimer (ECD).
Today (as of 2013) the method is rarely used outside of specialized centers, but it is of certain importance in the context of brain death diagnostics .
Basics
The brain is supplied with blood via the right and left internal carotid arteries and the basilar artery , which arises from the confluence of the right and left vertebral arteries . The above-mentioned vessels feed the cerebral arterial circle , from which the anterior cerebral arteries , the media cerebral arteries and the posterior cerebral arteries originate and supply the anterior middle and posterior parts of the brain (see also blood supply to the brain ). The complete failure of the blood supply to brain tissue leads within a few minutes to the irreversible destruction of the nerve cells in the affected area ( ischemic stroke ).
The function of the nerve cells in the brain is based on their electrical activity, the action potential , an energy-consuming process. In order to provide the necessary energy, nerve cells need oxygen (O 2 ) and glucose . The activation of certain areas of the brain results - through increased accumulation of carbon dioxide (CO 2 ) - in an expansion of the respective supplying arterioles and thus in an increased regional blood flow, whereby the substrates O 2 and glucose are available in sufficient quantities.
Indications and alternatives
Brain perfusion scintigraphy is used for the following indications , among others :
- Diagnosis and evaluation of cerebrovascular diseases
- Diagnosis and assessment of the course of suspected or proven dementia of the following forms: Alzheimer's disease , Lewy body dementia , dementia in Parkinson's disease , subcortical arteriosclerotic encephalopathy and frontotemporal dementia ; Even in the phase of mild cognitive impairment , abnormalities in the cerebral perfusion scintigraphy can provide information on the type and prognosis of the disease; as an alternative, positron emission tomography with 18 F- fluorodeoxyglucose is available
- Focus search in epilepsy (for example before epilepsy surgery ); Electroencephalography and functional magnetic resonance imaging (fMRI) are available as alternatives
- Evaluation of known or suspected traumatic brain injury ; Abnormalities in the brain perfusion scintigraphy with inconspicuous other imaging ( magnetic resonance tomography , cranial computed tomography ) provide information on the prognosis of neuropsychological symptoms
- Evaluation of suspected inflammation such as Rasmussen's encephalitis , viral encephalitis (for example herpes simplex encephalitis ), vasculitis (for example systemic lupus erythematosus , Adamantiades-Behçet's disease ) or HIV encephalopathy
- Evidence of cerebral circulation stoppage in the context of the determination of brain death ; as an alternative, transcranial Doppler sonography is available
- scientific questions of regional brain function; alternative methods include a. The positron emission tomography with 15 O -labeled tracers ( see below ) or with 18 F - fluorodeoxyglucose and the functional magnetic resonance imaging (fMRI) is used
Contraindications
A pregnancy is considered, with few exceptions as an absolute contraindication for nuclear medicine examinations, breast-feeding as a relative contraindication. After a scintigraphy with 99m Tc, the breastfeeding woman should express and discard the milk for 48 hours. An uncooperative or incapable of cooperating patient is also considered a contraindication for cerebral perfusion scintigraphy.
Radiopharmaceuticals
For scintigraphy and single-photon emission computed tomography (SPECT), which are available in the routine diagnostics of numerous hospitals, highly lipophilic substances are used as tracers , which have a high extraction rate into the brain. Examples of these are 99m Tc - ethyl cysteinate - dimer (ECD, trade name Neurolite) and above all 99m Tc- hexamethylpropyleneaminooxime (HMPAO, trade name Ceretec). After accumulation in the brain, these substances are converted within the nerve cells into a hydrophilic form that can no longer leave the respective cell. The enrichment - which mainly takes place within the first two minutes after the injection and remains largely constant for about four hours - corresponds to the product of the extraction rate and blood flow. Since the extraction rate in turn depends on the blood flow, the regional cerebral blood flow cannot be quantified with these methods, but only represented relatively or semi-quantitatively.
The regional cerebral blood flow can be quantified using 15 O -labeled tracers - for example 15 O-labeled water (H 2 15 O) or 15 O- butanol - as part of positron emission tomography (PET). It is usually specified in ml / 100 mg / min . Since 15 O has a half-life of only about 2 minutes, these tracers are only available in the immediate vicinity of a cyclotron and therefore mostly only available at research facilities.
Because of the longer half-life of 18 F (about 110 minutes), 18 F- fluorodeoxyglucose is available at significantly more centers. The regional consumption of gucose by the brain is comparable to the regional blood flow in many questions. However, deviations apply in particular to tumors and inflammations .
Investigation
preparation
In the run-up to the examination, it should be ensured that the patient can later lie down during the recordings, which last about half an hour. This can be limited in the case of cognitive disorders (dementia) as well as choreic movement disorders . If sedation is necessary for the recordings , this should only take place when the accumulation of the tracer in the brain has been completed. In general, substances that can influence the cerebral blood flow should be avoided before the examination. This also includes caffeine and ethanol . Tobacco smoking and the use of psychotropic drugs should be avoided.
application
The administration (application) of the tracer should take place under conditions that avoid activation of certain areas of the brain as far as possible: It is recommended to administer the radiopharmaceutical via a peripheral venous catheter that was placed about 10 to 15 minutes before the application. The room should be quiet and have subdued light to prevent activation of the visual cortex, for example . The patient should have their eyes open, should not wear headphones or hearing protection, should not speak, should not read, and should generally lie still.
Image acquisition and reconstruction
The patient should be positioned as comfortably as possible during the recordings, the head should be lightly fixed so that artifacts caused by movement are minimized. However, the comfort of the patient should be given priority over an optimal head position, since an uncomfortable position increases the likelihood of inadvertent movements. The distance between the head and the camera should be as small as possible for good spatial resolution. Theoretically, image acquisition can already begin about 2 minutes after intravenous administration of the tracer, since the fixation of HMPAO and ECD in the brain is then complete. This fixation remains almost constant over about 4 hours; the image acquisition should then be completed accordingly. The best image quality is achieved after 30 to 60 minutes (ECD) or 30 to 90 minutes (HMPAO). Since at least 5 million registered events are required for good image quality, a gamma camera with several heads is preferred . In the case of single-head cameras, the recording time must be extended accordingly. Depending on the number of camera heads, the recording time is typically between 20 and 60 minutes.
The reconstruction takes place in three spatial levels , if possible without tilting in the transverse level . Low-pass filters reduce the image noise , but worsen the image resolution . To assess deep structures of the brain such as the basal ganglia and the thalamus is an attenuation correction applied.
interpretation
With normal results, the accumulation of the tracer in the gray matter is two to three times higher than that in the white matter . The highest enrichment can be found in the thalamus , the striatum and the visual cortex . In young people, the accumulation in the prefrontal cortex is higher than in the parietal cortex , while the distribution pattern becomes more even with older people. A slight asymmetry is to be regarded as normal.
The image analysis is initially carried out visually ("with the naked eye"). The examiner should always select the settings for image display such as color scale and threshold value as uniform as possible in order to support the formation of engrams for normal and pathological findings. Knowledge of current structural imaging findings ( magnetic resonance tomography , cranial computed tomography ), and possibly also the image fusion with these recordings, simplifies the interpretation. The patient's medical history (including current and previous medication and previous traumatic brain injuries ) and the results of neurological , psychiatric and neuropsychological tests (such as the mini-mental status test ) should also be considered.
Partial volume effects can make interpretation difficult. A reduction in the density of nerve cells or a reduction in the activity of the nerve cells in a region appear - like the actually measured reduced blood flow - as reduced tracer accumulation.
The regional cerebral blood flow (rCBF) can be determined semi- quantitatively using simple methods such as the region-of-interest technique compared to the opposite side or compared to certain regions ( cerebellum , same hemisphere , entire brain) or with complex methods such as pixel-by-pixel three-dimensional mapping (for example, non-linear data transformation on Talairach atlases ) and determination of the statistical deviation from the mean value of a normal group corresponding to the patient's age. For this purpose, there are various software packages that carry out a sectional view or surface analysis. If several series of images were created, subtraction images can highlight pathological findings more clearly.
Internal brain atrophy is indicated by the increased distance between the thalamus and the caudate nucleus . External cerebral atrophy is an enlargement of the cerebral furrows (sulci) to the disadvantage of the cerebral convolutions ( gyri ). The cerebral cortex is generally inhomogeneous in cerebral atrophy. The comparison with a current magnetic resonance tomography of the patient can be helpful for the image interpretation.
Examination for special questions
Focus search in epilepsy
The intravenous injection of the radiopharmaceutical is carried out during the seizure (ictal), if the seizure frequency is high, an electroencephalogram (EEG) may be recorded. Image acquisition (SPECT) can be performed after the seizure, up to four hours after the event. The comparison with an examination in the seizure-free interval (interictal) - possibly as a subtraction recording - can be helpful for the interpretation.
Diagnostics of the cerebral blood supply reserve
Determining the cerebral blood flow reserve can be useful in the following situations: transient ischemic attack (TIA), stroke , narrowing or occlusion of the common or internal carotid artery ( carotid artery ), vascular anomalies , follow-up follow-up after surgery on the carotid artery , questions about the need for the system a shunt before endarterectomy of the carotids, determination of the blood supply reserve before and after surgery or stent angioplasty in the area of the vessels supplying the brain , differentiation between vascular and neural forms of dementia.
Acetazolamide - a carbonic anhydrase inhibitor - is administered intravenously ( off-label use ) about 10 minutes before the radiopharmaceutical is administered . When side effects are dizziness , tinnitus , paresthesia ago around the mouth, nausea and hypotension. The symptoms are usually mild and go away on their own. Absolute contraindications to the use of acetazolamide are allergies to sulfonamides and the subacute stage of stroke . The relative contraindications are migraines , renal insufficiency and liver insufficiency . Acetazolamide increases arterial carbon dioxide levels . In healthy brain tissue, this leads to an expansion (dilatation) of the arterioles and a significant increase in blood flow. In those brain areas whose upstream vessels have constrictions, the arterioles are already maximally expanded to compensate for this, so that an increase can no longer take place here and the affected areas are shown in the scintigram as having less storage.
The comparison with a series of images without the use of acetazolamide - possibly as a subtraction image - can be helpful for the image interpretation.
Brain death diagnostics
The brain perfusion scintigraphy is used as part of diagnostics to determine brain death . The proof of the loss of cerebral blood flow by means of cerebral perfusion scintigraphy or transcranial Doppler sonography proves the irreversibility (irreversibility) of the brain function failure without further observation time, which is necessary, for example, with an exclusive clinical-neurological examination and which can be up to 72 hours in children and infants.
The following findings are considered to be scintigraphic criteria for brain death: lack of representation of the cerebral vessels, lack of representation of cerebral blood flow, lack of accumulation in the brain tissue ( empty skull ). There is a redistribution of the blood flow in the flow area of the external carotid artery and an increased accumulation of the tracer in the area of the nose ( hot nose ).
The German guidelines for brain death diagnostics require the use of hexamethylpropyleneaminooxime (HMPAO). A radiochemical quality control should be carried out and documented. The radiochemical purity of the preparation must be at least 90%. If two-dimensional (planar) recordings are available from different viewing directions (projections), a three-dimensional representation (SPECT) can be dispensed with. Additional planar images of the chest and abdomen are recommended in order to document normal distribution of the tracer as an in vivo quality control.
Activities used and radiation exposure
The guidelines of the German Society for Nuclear Medicine and the European Association of Nuclear Medicine (EANM) provide for activities of up to 1100 MBq to be administered for the 99m Tc -labelled radiopharmaceuticals . Significantly lower activities can lead to poor image quality, especially when using high-resolution collimator systems (for example in the context of the focus search before epilepsy surgery).
As radiation exposure are for 99m Tc Ethylcysteinat - dimer (ECD) from 0.0077 to 0.011 mSv / MBq (adults) and 0.023 mSv / MBq (5-year-old child) and 99m Tc Hexamethylpropylenaminooxim (HMPAO) 0.0093 mSv / MBq (Adults) and 0.026 mSv / MBq (5-year-old child). The "critical organ" - the organ that receives the highest organ dose - is the urinary bladder wall for ECD and the kidney (adult) or thyroid (5-year-old child) for HMPAO .
The radiation exposure when using 15 O-labeled water in positron emission tomography (PET) is given as 0.00093 mSv / MBq. With an administered activity of 1,000 MBq, the effective dose is 0.93 mSv.
outlook
Simplified image interpretation and improved image quality can be expected from the increasingly widespread combination of single-photon emission computed tomography and computed tomography (SPECT / CT). The attenuation correction that occurs in principle with this combination method is advantageous . Another method of simultaneous image acquisition with two methods ( modalities ), which (as of 2013) has not yet become widespread, is the combination of magnetic resonance tomography and SPECT (MRI / SPECT). The (almost) simultaneous collection of structural and functional image data in the same position of the patient makes the subsequent image fusion, which is fundamentally faulty due to the lack of anatomical landmarks , superfluous. Tasks for the future are the collection of meaningful normal collectives - in principle for every age group and every camera system - and the improvement of the methods for quantifying the findings.
literature
- Torsten Kuwert: Brain. In: Torsten Kuwert, Frank Grünwald , Uwe Haberkorn , Thomas Krause : Nuclear Medicine . 4th, newly created and expanded edition. Thieme, Stuttgart et al. 2008, ISBN 978-3-13-118504-4 , p. 231-257 .
- Christian Menzel, Peter Bartenstein a. a .: Guideline for brain perfusion SPECT with technetium-99m radiopharmaceuticals. ( Online at the German Society for Nuclear Medicine , nuklearmedizin.de)
- Özlem L. Kapucu, Flavio Nobili et al. a .: EANM procedure guideline for brain perfusion SPECT using 99mTc-labeled radiopharmaceuticals, version 2. In: European Journal of Nuclear Medicine and Molecular Imaging. 36, 2009, pp. 2093-2102, doi: 10.1007 / s00259-009-1266-y .
- Scientific Advisory Board of the German Medical Association : Guidelines for determining brain death. Deutsches Ärzteblatt 95, issue 30, July 24, 1998 ( bundesaerztekammer.de PDF, 103 kB).
Individual evidence
- ↑ Der Kleine Stowasser , Munich 1971
- ↑ G. Hoglinger, Stefan Kleinert: Brain death and organ transplantation . Walter de Gruyter, 1998, ISBN 978-3-11-016203-5 ( limited preview in Google book search).
- ↑ Özlem L. Kapucu, Flavio Nobili et al. a .: EANM procedure guideline for brain perfusion SPECT using 99mTc-labeled radiopharmaceuticals, version 2. In: European Journal of Nuclear Medicine and Molecular Imaging. 36, 2009, pp. 2093-2102, doi: 10.1007 / s00259-009-1266-y .
- ↑ Torsten Kuwert: Brain. In: Torsten Kuwert, Frank Grünwald , Uwe Haberkorn , Thomas Krause : Nuclear Medicine . 4th, newly created and expanded edition. Thieme, Stuttgart et al. 2008, ISBN 978-3-13-118504-4 , p. 237 .
- ^ W. Sonnenschein, A. Bockisch: Radiation protection. In: Torsten Kuwert, Frank Grünwald , Uwe Haberkorn , Thomas Krause : Nuclear Medicine . 4th, newly created and expanded edition. Thieme, Stuttgart et al. 2008, ISBN 978-3-13-118504-4 , p. 71 .