Positron emission tomography
The positron emission tomography ( PET ; of ancient Greek τομή tome , cut 'and γράφειν graphein , write') is a imaging technique of nuclear medicine .
It is a variant of emission computed tomography . PET creates sectional images of living organisms by making the distribution of a weakly radioactively marked substance ( radiopharmaceutical ) visible in the organism and thus depicting biochemical and physiological functions ( functional imaging ). It is based on the simultaneous detection (detecting) of two gamma-ray photons , which after the decay of positron emitting (emitting) radionuclide formed ( β + -decay ). Today, PET is almost exclusively carried out together with a CT or MRT as a hybrid procedure.
principle
PET
Based on the principle of scintigraphy , the patient is given a radiopharmaceutical at the beginning of a PET examination , usually by injection into a vein in the arm . Radiopharmaceuticals are drugs used as trace substances which are used below a physiologically effective dosage and which contain radionuclides as an essential component that emit positrons ( radiation ). The positrons emitted in the body only have a lifespan in the range of nanoseconds and interact with an electron almost instantaneously. This creates a secondary annihilation radiation ( annihilation ), in which two high-energy photons are (energy per 511 keV) in exactly opposite directions, ie each other at an angle of approximately 180 degrees. The PET device contains many detectors for the photons , mostly arranged in a ring around the patient . Thanks to fast electronics, only those decay events are counted which are based on exact coincidences between two opposing detectors. From the temporal and spatial distribution of these registered decay events, conclusions are drawn about the spatial distribution of the radiopharmaceutical inside the body and a series of cross-sectional images is calculated.
In contrast to the transmission-based tomography methods , the image creation with PET and SPECT is based on emission and thus depicts the distribution of the previously injected radiopharmaceutical in the body. This results in a primarily functional mapping and only secondarily, depending on the function shown, a morphological mapping. Therefore, the importance of using PET and the like is a. in answering questions about metabolic processes (e.g. glucose metabolism ), receptor status (e.g. dopamine receptors ), or surface antigen properties (e.g. PSMA status). Important clinical applications are in oncology , neurology and cardiology .
Combined PET / CT devices for human diagnostics in research and clinics are offered by Canon Medical Systems , General Electric , Hitachi , Mediso , Philips and Siemens Healthineers ; Devices only suitable for PET were only built until 2003.
Comparison with SPECT
In single-photon emission computed tomography (SPECT), a collimator is required to determine the beam direction of the photons to be measured . Since this fades out a large part of the photons produced, only about 1 ten thousandth of the emitted photons can be detected. With PET, on the other hand, physical collimation can be dispensed with due to the measuring principle of the coincidence detection, which leads to a yield improved by a factor of 100, higher counting rates and thus improved image statistics with higher image quality and increased spatial resolution. Since the absorption of the photons only depends on the thickness of the irradiated tissue, but not on the location of the photons, this also enables an exact quantification of the tracer distribution in the examination volume, which is not possible with SPECT.
Radionuclides
The most commonly used nuclide for radiopharmaceuticals in PET is the radioactive isotope 18 F of fluorine . It is produced with the help of a cyclotron and, due to its half-life of around 110 minutes, can also be used if there is no cyclotron at the examination site and the radiopharmaceutical must first be transported from the production site to the examination site. For this reason, it is used in over 90% of all PET examinations.
| nuclide | Half-life |
|---|---|
| 11 C | 20.3 minutes |
| 13 N. | 10.1 minutes |
| 15 O | 2.03 minutes |
| 18 F | 110 minutes |
| 68 Ga | 68 minutes |
| 82 Rb | 75 seconds |
In addition to 18 F, 11 C , 13 N , 15 O , 82 Rb or 68 Ga are mainly used. These are radioactive isotopes of the elements carbon , nitrogen , oxygen , rubidium and gallium .
68 Ga and 82 Rb are traditionally used as generator radioisotopes. The radioisotope is created by the decay of an unstable parent isotope in a so-called radionuclide generator , in which it accumulates (→ Gallium-68 generator ). Only more recent developments allow 68 Ga to be produced in specially designed cyclotron facilities. All other PET nuclides mentioned are produced exclusively with the help of a cyclotron .
The radionuclide used has an influence on the critical performance parameters of the PET device: While short-lived nuclides require that the PET scanner can process the associated high counting rates with activities of several gigabecquerels (GBq), at 18 F, mostly in the form of Fluorodeoxyglucose applied, the sensitivity of the system required, since the injected activity here is mostly only between 100 and 350 megabecquerels (MBq).
The meaning of the half-life should be explained using a calculation: After 60 minutes, only about one eighth of an initial activity of 1000 MBq 11 C is left. For an 11 C-based radiopharmaceutical, 60 minutes is roughly three half-lives (exactly: 60 / 20.3 = 2.956). In this example, the remaining activity after one hour is 1000 MBq × 1/2 2.956 = 128 MBq. Using 11 C therefore requires that a cyclotron be in relative proximity to the PET system. If 13 N or 15 O are used, the cyclotron must be in the immediate vicinity of the PET scanner. A radiopharmaceutical production plant with a cyclotron requires an investment in the tens of millions, which limits the use of the nuclides mentioned for PET to appropriately set up centers.
Radiopharmaceuticals
Radiopharmaceuticals are drugs that are marked with a radionuclide and that the organism cannot distinguish from their non-radioactive counterparts . They therefore take part in the normal metabolism , but are used in a concentration that does not influence the metabolism to be measured quantitatively ( tracer principle). Due to its radioactive decay, the amount and distribution of the radionuclide in the body can be measured and displayed.
Enrichment Mechanism
The Nobel laureate Otto Warburg recognized as early as 1930 that tumor cells, due to an increased metabolism, usually consume more glucose than normal body cells ( Warburg effect ). 18 F- fluorodeoxyglucose (FDG) is taken up by cells via the glucose transporter in exactly the same way as glucose , although a hydroxyl group has been replaced by the radionuclide 18 F at one point on the molecule . Since the FDG-6 phosphate, which is produced intracellularly from the phosphorylation of FDG in the next metabolic step , is not further metabolized, an accumulation takes place (“ metabolic trapping ”), which is proportional to the current metabolic state of the cells. This is particularly advantageous for the early diagnosis of cancer if no tumor-related increase in size has yet taken place, which is necessary for detection in other sectional image methods. In addition to finding tumors and metastases, the distribution of FDG in the body also allows general conclusions to be drawn about the glucose metabolism of tissues. The enrichment principle of other radiopharmaceuticals obeys similar mechanisms: Wherever the radiopharmaceutical is enriched, this becomes visible through increased radioactive decay at this point in the PET image. Choline is required for the biosynthesis of cell membranes . Choline is phosphorylated in the cells and incorporated into phospholipids as phosphorylcholine . Tumor cells have an increased need for choline because they multiply more quickly. [ 18 F] choline and [ 11 C] choline are used as choline tracers . Thymidine tracers are suitable for marking DNA that is increasingly duplicated by tumor cells. For this purpose, 14 C-, 3 H-, 11 C- and 18 F-labeled thymidine are used, which are incorporated into the DNA like natural thymidine.
Typical areas of application for selected radiopharmaceuticals
| nuclide | Radiopharmaceutical | field of use |
|---|---|---|
| 11 C | [ 11 C] - choline | Prostate cancer diagnostics (has largely been replaced by 18 F-choline) |
| [ 11 C] Pittsburgh compound | Pittsburgh compound B for early Alzheimer's diagnosis (still in development) | |
| [ 11 C] -S-methyl-L- methionine | Visualization of protein biosynthesis (diagnosis of low-grade malignant gliomas ) | |
| [ 11 C] acetate | Visualization of the oxygen consumption of the heart muscle | |
| 13 N. | [ 13 N] L-glutamic acid | Representation of the amino acid metabolism |
| [ 13 N] ammonia | Illustration of the heart muscle blood flow | |
| 15 O | [ 15 O] water | Depiction of blood flow ( perfusion ) |
| [ 15 O 2 ] | Representation of oxygen uptake and distribution | |
| 18 F | [ 18 F] choline | Prostate Cancer Diagnostics |
| [ 18 F] PSMA-1007 | Prostate cancer diagnostics; has a greater sensitivity than F18 choline | |
| [ 18 F] - fluorouracil | Representation of tumors and for therapy control | |
| [ 18 F] - fluoroethyl tyrosine | Representation of brain tumors (in combination with [ 11 C] -S-methyl-L-methionine) | |
| [ 18 F] - sodium fluoride | Illustration of the bone metabolism | |
| [ 18 F] - 2-fluoro-2-deoxy-D-glucose | Representation of glucose transport and glucose turnover | |
| [ 18 F] -6-fluoro- DOPA | Presentation of the presynaptic dopamine pool for diagnosing neuroendocrine tumors ( e.g. medullary thyroid carcinoma ) | |
| [ 18 F] -Florbetaben, -Florbetapir, -Flutemetamol | Amyloid-beta tracer for early detection of Alzheimer's disease (the radio-pharmaceutical amyvid has been approved in Europe since the beginning of 2013) | |
| 68 Ga | DOTATOC | Somatostatin receptor imaging of neuroendocrine tumors: pancreatic tumor , meningioma , small cell lung cancer or carcinoid |
| 82 Rb | [ 82 Rb] chloride | Illustration of the heart muscle blood flow |
The PET examination
Indications
Because of the high costs and radiation protection , PET examinations should only be carried out if the benefit is proven. For example, the German Society for Nuclear Medicine assesses in detail for which indications PET is useful . Internationally accepted indications are currently:
| illness | Assumption of costs for |
|---|---|
| Solitary pulmonary nodule | characterization |
| non-small cell lung cancer | characterization |
| Esophageal carcinoma | Primary staging , diagnosis, staging , re-staging |
| Colorectal cancer | Diagnosis , staging and re-staging |
| increasing CEA value | Tumor localization |
| Lymphoma | Diagnosis, staging and re-staging |
| Melanoma | Diagnosis, staging and re-staging |
| Breast cancer | Restaging, therapy progress or discussion of a
Change of therapy regimen for metastatic breast cancer |
| Head and neck cancer | Diagnosis, staging and re-staging |
| follicular thyroid carcinoma | Re-staging of the recurrence or residual tumor |
| Basal ganglia diseases | early differential diagnosis of Parkinson's disease , early diagnosis of multisystem degenerations, |
| dementia | Early diagnosis of primary dementia |
| epilepsy | Localization of the epileptogenic focus during the preoperative
Epilepsy diagnostics in temporal lobe epilepsies (with F-18-FDG) |
PET testing during chemotherapy may be beneficial for patients with Hodgkin's lymphoma. When comparing PET-negative (= good prognosis) and PET-positive (= bad prognosis) patients, Aldin et al. the following results: The evidence is very uncertain about the effect of the effect of negative and positive interim PET studies on progression-free survival over an observation period of 3 years. Negative interim PET exams may result in an increase in progression-free survival compared to positive exam results when the adjusted effect is measured. Negative interim PET results can lead to a marked increase in overall survival compared to positive results if the observation period is 3 years and also if the adjusted effect is measured.
procedure
The test person receives the radiopharmaceutical by injection or inhalation. In the case of an FDG-PET examination, 150 to 700 MBq are injected depending on the patient's weight and device (2D or 3D scanner); then the patient has to rest for about 50 to 75 minutes so that the tracer has enough time to accumulate in relevant places in the body ( uptake phase). With FDG-PET, the patient should be fasted at the time of the examination. During the examination, he should lie quietly and not freeze, otherwise the increase in sugar metabolism would be visible as an accumulation in the muscles and brown adipose tissue . During the examination, the patient is positioned on a movable table in such a way that the body section to be examined is in the field of view of the detectors.
The axial field of view of the detectors - also called Field of View (FOV) - is around 15 to 20 cm in commercial systems. For recordings that cover a larger part of the body, it is therefore necessary to record several so-called bed positions. Depending on the device type, the overlap between the bed positions varies and is between around 1.5 and 5 cm. For a full-body exposure, there are therefore around 8 to 12 bed positions. Usually, however, only partial body recordings are carried out. Depending on the device, tracer used, injected dose and patient's body weight, the recording time is two to four minutes per bed position. After this time has elapsed, the device automatically moves to the next bed position.
With PET / CT, the patient is driven through both detector rings ( gantries ) of the CT and PET immediately one after the other . Since the CT data set is required for the reconstruction of the PET data, a CT recording is usually first carried out and the PET recording is then connected. In this way, the image reconstruction can be started after the first bed position has been recorded. If the PET data set were to be recorded first, the start of the image reconstruction of the PET data would have to wait until the CT data set has been reconstructed and this would lead to undesirable delays in the workflow, because the reconstruction of a whole-body PET data set requires, depending on the device and calculation method approx. 10–45 minutes. A running CT recording can be recognized by the patient by the fact that the table is being moved while a motor noise can be heard at the same time, which primarily comes from the rotational movement of the tube and detector system. In some systems, the gantries are housed in the same housing and are not individually visible from the outside. The calculated images are automatically merged in the computer .
With PET / CT devices, the CT recording is absolutely necessary for the calculation of attenuation-corrected PET images, but a so-called low-dose CT scan is sufficient for this. With some devices, any CT protocol, including a diagnostic CT recording, can be used to calculate the attenuation map; some devices require the additional performance of a dedicated low-dose scan, even if the patient already has a high-dose CT image.
Diagnosis
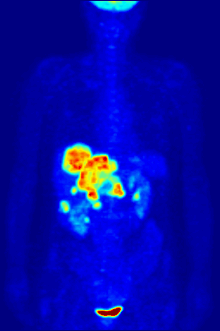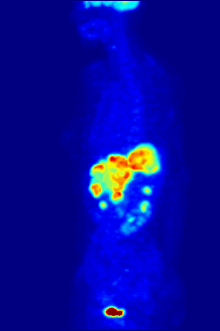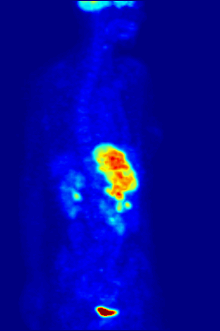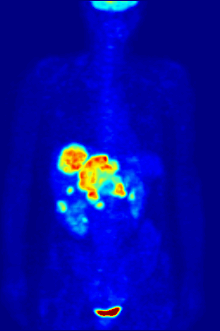
The diagnosis of PET or PET / CT image is carried out by viewing the reconstructed slice images (axial, coronal or sagittal). In PET / CT devices, the display of PET and CT is linked so that the position of the correlating PET image is automatically shown when the image cursor is moved in the CT image.
A so-called MIP ( Maximum Intensity Projection ) image is used for an overview . In this representation, the entire object to be examined is shown and a quick overview of regions of increased exposure is obtained. For even better visualization, the MIP can be rotated around itself in order to show the examination object from all sides.
Regions with increased uptake are quantified using the so-called Standardized Uptake Value , or SUV for short. The SUV value describes the nuclide accumulation taking into account the nuclide decay, the administered dose and the patient's weight in order to obtain a quantification independent of time and weight.
When assessing enrichment, a distinction must be made between physiological and pathological uptake. An uptake can have very different causes: a tumor can lead to FDG accumulation, as can the healing of a wound, an inflammation process, simple muscle tension or a freezing patient.
The so-called Total Lesion Glycolysis (TLG) is derived from the SUV, it is the mean SUV value times the tumor volume and, in contrast to the SUV, does not describe the maximum or mean glucose turnover at a specific point, but the glucose turnover of the entire lesion.
Radiation exposure
Since PET is based on the administration of a radioactive substance, the indication must be made cautious, as with all imaging methods that work with ionizing radiation (e.g. CT, angiography ).
The radiation exposure of a pure PET examination with [ 18 F] -FDG is around 7 mSv for PET when 350 MBq are injected and between 3 and 10 mSv for CT. The radiation exposure from the CT depends essentially on whether it is only to be used for localization or also for diagnostics. The radiation exposure from PET alone is thus in the order of magnitude of a computed tomography of the thorax. It should be noted, however, that the reference dose of older 2D scanners used up to around the year 2000–2005 for a 70 kg patient is 370 MBq, while that of today's 3D scanners is 200 MBq.
As with all examination methods with ionizing radiation, the amount of radiation in PET must be related to the information obtained. With a radiation dose of 1 Sievert (Sv) to which 100 people are exposed, 5 deaths from radiation cancer are to be expected; for those over 60, a value of 1.2 deaths per Sievert applies. So it would take 100,000 PET scans to cause 35 deaths from radiation cancer (after a mean latency of about 15 years for leukemia and about 40 years for solid tumors), that is, about one in 3000 examinations.
It must be taken into account that the majority of PET examinations are carried out on oncological issues, i.e. in patients with, in some cases, significantly reduced life expectancy.
For example, a PET scan is performed for staging a patient with non-small cell lung cancer ( five-year survival rate of 15%). Here the theoretical risk for the patient of developing a radiation-induced second tumor 20 or 30 years later is negligible. In contrast, in 11 to 37% of cases with this disease, the PET examination leads to a significant change and thus usually to an improvement in the therapeutic procedure.
In order to reduce the radiation exposure for children with cancer, pediatric radiologists and nuclear medicine specialists at Leipzig University Hospital recommend the combined diagnostics of PET and MRI. With the same informative value, this is gentler, even if it is only available in seven centers across Germany.
Costs and assumption of costs
PET is one of the most expensive imaging procedures in modern medicine. The cost of a PET examination can be up to 1500 euros. For example, a full-body PET / CT costs around 1,123 euros in Germany ( as of April 2008 ). This is roughly twice the price of a full-body MRI at 575 euros. The pure device costs for a PET device are between 1.5 and 3 million euros, depending on the equipment.
A large part of the radiopharmaceuticals necessary for diagnostics has to be produced using a cyclotron , which causes high costs in the manufacturing company. Since the production of radiopharmaceuticals is subject to the Medicines Act, all the requirements and laws to which a pharmaceutical manufacturing company is subject apply, so that the total investment for the production of radiopharmaceuticals is around ten million euros.
In contrast to the practice in other European countries, the statutory health insurance in Germany usually only covers the costs for a PET examination if the patient is admitted or treated as an inpatient. However, the medical service of the health insurance companies increasingly refuses to accept the costs if the patient was only admitted to the hospital for PET / CT, i.e. if no other diagnostics were carried out or if therapy was not started immediately afterwards.
Since 2007, the costs of PET examinations for the diagnosis of non-small cell lung cancer (NSCLC) have been covered by the statutory health insurance companies in Germany, but initially there were no EBM numbers, so that routine accounting was difficult. Since January 1, 2009, small cell lung carcinoma (SCLC) has been added to NSCLC. In the event of an unclear residual tumor, the joint federal committee has introduced the 18 F-FDG PET / CT as a standard benefit in the outpatient area of the statutory health insurance companies.
Since October 2010, the costs for PET diagnostics have also been covered for malignant lymphoma under certain conditions.
Since January 1, 2016, the statutory health insurance (GKV) in Germany has been reimbursing the costs for a PET or PET / CT examination as a new statutory health insurance service for the above-mentioned indications. The EBM has been expanded to include a new section 34.7 with the fee schedule items (GOP) 34700 to 34703. On November 2, 2017, quality assurance was adapted to PET and PET / CT in an agreement.
Sensitivity and specificity of PET in diagnostics
In the beginning, sensitivity and specificity information often referred to studies that were still carried out with pure PET scanners. Pure PET devices had both a poorer image resolution (5–8 mm) and a poorer image quality (image noise) than the PET / CT devices available today, which achieve resolutions of less than 4 mm.
The image quality is also influenced by the time required for the examination. With a pure PET scanner you need up to 90 minutes for an image, a modern PET / CT scanner only needs approx. 15 minutes for a better quality image. If the patient moves during the exposure, the image will be blurred. False negative results are often due to the fact that the lesion is smaller than the device can display. A major problem at the time of the pure PET devices was also to distinguish physiological tracer accumulations from pathological accumulations, since the unambiguous anatomical assignment of an accumulation was not possible or only with difficulty. The introduction of PET / CT brought great progress here. The specificity of PET diagnostics requires organ-specific tracers. Since these can usually not differentiate between inflammatory and degenerate tissue, a histologically confirmed finding of the primary tumor must be available before a metastasis search using PET.
Lung cancer
In the majority of the studies carried out, PET (NOT PET / CT) achieved an overall sensitivity of about 92% in the primary diagnosis of lung cancer with a specificity of 90% in the detection of foci larger than 7 mm. False negative diagnoses are mostly due to a high serum glucose level and sometimes occur in small herds that do not give a clear SUV value due to breathing movements in the tip of the lungs.
Breast cancer

The performance of PET / CT in breast cancer diagnostics depends on the histological type and size of the lesion being detected. Tumors of lobular histology, tubular carcinomas, and small (<1 cm) in-situ carcinomas show a low level of FDG uptake and are therefore more difficult to detect. The available resolution of the recording device is also a critical parameter here.
For tumors larger than 1 cm, as well as for the detection of affected lymph nodes, a sensitivity of greater than 90% is reported. However, PET seems to underestimate the number of affected lymph nodes; the sensitivity also depends on the total tumor burden. A large study reports only 60% sensitivity with 80% specificity in the detection of axillary lymph nodes.
PET / CT plays a major role in monitoring the progress (staging) of ongoing chemotherapy. It is also recommended for radiopaque or large breasts, which cannot be adequately assessed with other methods, for finding and assessing (bone) metastases and for diagnosing recurrences .
Colon cancer
The detectability of tumors and metastases of colon cancer is impaired by the fact that FDG accumulates in the intestine anyway due to physiological processes, which worsens the signal-to-noise ratio . Here, PET / CT has brought about a significantly better diagnosis compared to pure PET, as it has greatly improved the anatomical assignability of enrichments.
Meta-studies have shown that PET / CT is superior to CT, MRI and ultrasound in terms of sensitivity and specificity. One study reported 94% sensitivity and 87% specificity. In patients with a rise in CEA, in whom computed tomography did not enable the tumor to be localized, PET showed a sensitivity of over 80%. A clear strength of PET lies in the early diagnosis of the response to chemotherapy, where it is able to classify " responders " (tumor responds to chemotherapy) and "non-responders" very early on.
Head and neck cancer
In some studies, sensitivities of 90 to 100% were given for known tumors. False negative results were obtained in very small lesions and in mucus-forming tumors. In the case of salivary gland tumors, the sensitivity is less than 70%. PET is inferior to MRI here. When staging lymph node metastases with known head and neck cancer , PET is clearly superior to MRI (90% sensitivity vs. 75%). PET is the most suitable diagnostic method for restaging head and neck tumors (for evaluating therapy response).
False positive results result from lymph nodes in the head and neck region that show an inflammatory reaction as part of therapy. “Physiological” (normal) FDG uptake also takes place in the head and neck area (for example in the salivary glands). Therefore - as with colon cancer - there is a fundamental risk of mistaking physiological accumulations (false positive) for tumor-associated and accumulations in tumor tissue wrongly (false negative) for physiological accumulations.
Skin cancer (such as malignant melanoma)
In the literature, sensitivities of PET (not PET / CT) of 80–100% are given, with false negative results being observed for very small lesions (<3 mm). The sensitivity of the method is thus limited by the image resolution of the PET scanner. The specificity is given as about 80%. PET (/ CT) is very suitable for the detection of metastases. Skin cancer is one of the most metabolically active tumor entities and for this reason often shows very high levels of FDG.
Prostate cancer
In a study in which 67 patients with histologically confirmed prostate cancer participated, the results of the PET / CT were correlated with the histological findings. A sensitivity of 11 C-choline PET / CT for finding lymph node metastases of 80%, a specificity of 96%, and an accuracy of 93% were stated. A new tracer for diagnosing prostate cancer has been available since around 2013. The prostate-specific membrane antigen (PSMA) is coupled to the radionuclide 68 Ga.
Bone metastases
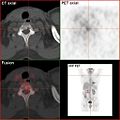
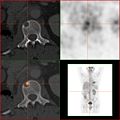
Bone metastases of various tumor diseases can be detected very well with PET / CT. The method is more sensitive than SPECT and considerably more sensitive than planar skeletal scintigraphy , but it is also more complex and therefore more expensive. Depending on the tumor entity, different tracers are used, but mostly FDG and sodium fluoride, in prostate cancer also 11 C or 18 F choline. Metabolic activity of the tumor can be visualized with the help of the tracer FDG, the reaction of the bone to the tumor with the sodium fluoride PET / CT (see picture examples).
HDP skeletal scintigram of a patient with kidney cancer : The bone metastases in the cervical vertebrae 7 and in the lumbar spine (LWK 1 & 2) can only be guessed at due to the low resolution of the planar skeletal scintigraphy.
Sodium fluoride PET / CT of the same patient: The osteolytic bone metastasis in the cervical spine shows an extreme uptake and is clearly visible. The two other bone metastases in the lumbar spine are also shown very well in the PET / CT image.
A false positive, diffuse FDG uptake can be observed in the bone marrow as a reaction to a recently performed chemotherapy. In the case of PET imaging with sodium fluoride (NaF), it should be noted that even after successful therapy, an accumulation can be observed in the bone, which in this case does not represent the activity of the tumor but the repair activity of the osteoblasts (bone-forming cells).
History of PET and outlook for future developments
The invention of PET is commonly attributed to the American physicists Michel Ter-Pogossian and Michael E. Phelps , who published their results in 1975. The foundations were laid by the neuroscientist Louis Sokoloff , who together with Martin Reivich showed in the early 1970s that radioactive glucose analogues were well suited for recording brain activity. He used the isotope carbon-14, Alfred P. Wolf proposed the isotope fluorine-18 instead in the tracer FDG (synthesized by Wolf and Joanna Sigfred Fowler ). The substance was first used in 1976 by the neuroscientist Abass Alavi (* 1938) for a PET scan of human brains.
Gordon Brownell and WH Sweet localized a brain tumor at the Massachusetts General Hospital in the early 1950s with the help of positron-based imaging technology and a computer-aided image reconstruction based on an attenuation-corrected, filtered back projection was created by David Chesler there in 1970.
From PET to PET / CT
PET is a highly sensitive process when it comes to the right question; However, it is not always easy to localize activity accumulations anatomically , since primarily metabolic processes are shown in PET images; In addition, there is the limited spatial resolution of around 4–6 mm.
A PET / CT device combines the high spatial resolution (of up to 0.35 mm) and detailed anatomical representation of the CT device with the highly sensitive metabolic information from the PET.
Devices that combine a PET scanner with a computer tomograph (CT) have therefore been on the market since 2001 . The world's first device of this type was installed by the General Electric company at the University of Zurich in March 2001, while the first device in Germany was put into operation by Siemens at the University Hospital Essen at the end of 2001.
In the case of PET / CT systems, the correction maps required to correct the attenuation of the PET data are calculated from the Hounsfield values of the CT data. With pure PET scanners, a separate radiation source was required for this, with the help of which this attenuation map was created. Since the CT scan is recorded much faster than the previously required transmission recording with a radiation source, this results in a significant reduction in the recording time of up to 40%. The examination time for whole-body images is in the range of 15 to 30 minutes with PET / CT systems.
PET / CT devices for clinical use have completely displaced pure PET scanners on the new device market as early as 2004.
Since some older pure PET scanners are still in use (see below), an overlay (so-called soft fusion or co-registration ) of CT images and PET data is also calculated using software. This is done with the help of common reference points such as bone structures or external position data. The disadvantage of the subsequent fusion of the image data is that the CT image used for the image fusion was not used to reconstruct the PET data (from a pure PET scanner), and the image quality of the resulting PET / CT image is therefore worse than that of a dedicated PET / CT is.
An image fusion of PET images with MRI images is also possible, which u. a. is used in radiation therapy .
Time of Flight
This method for increasing the signal-to-noise ratio has been researched since the early 1980s . In 2006, Philips was the first manufacturer to use this measuring principle for the Gemini-TF in a clinical PET / CT system for human diagnostics. At EANM 2008, Siemens presented the Biograph mCT, a PET / CT system for which the TOF measurement was available from the end of 2009. At the SNM 2009, General Electric also presented the Discovery 690 , a device that uses time-of-flight technology.
Hybrid device made of PET and MRI
As a relatively recent development, the combination of PET and MRT in one device has been established as a new diagnostic method for clinical use. One of the first hybrid devices made of PET and MRT with a magnetic flux density of 3 Tesla was installed at Forschungszentrum Jülich in 2007 ; In 2009 a hybrid device with a flux density of 9.4 Tesla was put into operation there. Such a PET / MRT hybrid device that is used for brain diagnostics has also been in use at the University of Tübingen since mid-2007. The next step, namely the use of the combined PET / MRT also for whole-body examinations, was taken by Philips and Siemens, who showed the corresponding devices for the first time at the RSNA 2010 and installed them in Munich, Tübingen and Dresden. They enabled combined PET / MRT examinations through the complete integration of PET and MRI (Siemens) or through the use of a shared patient bed, which was mounted between the two tomographs installed at a distance of almost 3 meters (Philips). This patient couch positioned the patient successively in both tomographs. The overall system then enabled combined PET / MRT examinations through the use of appropriate software without the need to reposition the patient. The small time lag inherent in this procedure (about 10 minutes) between the PET and MRT examinations (sequential imaging) was considered irrelevant for many questions, especially in the oncological field. For special neurological and cardiological questions in particular, however, it is advantageous to carry out both examinations as synchronously as possible, as is possible with a fully integrated PET / MR device. In the meantime, the partially integrated device concept has been abandoned; the second-generation PET / MR devices currently available from Siemens Healthineers and GE Healthcare are fully integrated.
In the case of simultaneously measuring PET / MR systems, the photomultipliers normally used in PET scanners cannot be used due to the magnetic field required for MR imaging; Avalanche photodiodes or silicon photomultipliers ( SiPM ) are therefore used to detect the PET signals . Due to their physical properties, silicon photomultipliers are also suitable for the use of time-of-flight technology.
The CT image data of a PET / CT are among other things the basis for the scattered radiation and absorption correction. Hounsfield units are ultimately attenuation units that can be assigned to the absorption coefficients of the 511 keV photons using lookup tables .
Challenges in the construction of a PET / MR device therefore exist. a. also in deriving this information from an MR image and shielding the PET radiation without impairing the performance of the overall system.
The combination of the CT modality with the PET modality led to a drastic reduction in the total recording time, the image quality of the PET recording to be improved considerably, and the superposition of the two images in the main clinical area of PET application - oncology - meant a significant diagnostic added value.
The acquisition of MRT image data requires a considerably longer acquisition time than computed tomography, so it was initially assumed that the combination of PET / MR would represent a step backwards compared to PET / CT due to the associated extension of the examination and would impair its use in practice. With simultaneous recording technology, however, the time required for the MRT also increases the recording time that can be used for the PET, which can be used to significantly improve the quality of the PET and also allows statements to be made about the time-dependent distribution in the imaged volume. In addition, methods have been developed in which the location coding recorded in the MRT is used to correct the movement of the PET images. Advances in the development of sequences in MRI for whole-body recordings have made it possible to reduce the relative disadvantage with regard to the total recording time. In particular for questions for which an MRI is indicated and planned anyway, the combined examination offers a time and cost advantage compared to separate examinations. Since the integrated PET / MR, in contrast to PET / CT, enables a simultaneous examination of both modalities, there is a clinically relevant advantage here, especially when imaging moving organs (heart, lungs, liver, intestines).
Research topics

Despite the long history of PET, work is being done around the world to improve the process.
Current research:
- Due to the relatively long recording times, which are 15 to 30 minutes even with modern devices, the movement of the patient in the scanner cannot be prevented. In the area of the thorax there is practically always movement due to breathing and heartbeat . Because of the steadily increasing spatial resolution of new positron emission tomographs, this problem is becoming more and more noticeable, because the images look blurred in places . There are therefore more and more research groups around the world that are devoting themselves to this topic and are trying to record the patient's movement during the examination with the help of motion detection systems (so-called motion tracking) in order to subsequently modify the raw data of the recording so that cross-sectional images are almost completely free of movement can be generated. There are also some methods that manage entirely without motion detection systems.
- In the case of combined simultaneous PET / MRT imaging for small animals, research is currently being carried out on new detector technologies and integration concepts. The aim here is to increase the spatial and temporal resolution, the sensitivity and avoid the interference that occurs in the case of simultaneous PET-MRT imaging. The research of the EU-FP7-funded projects HYPERImage, SUBLIMA and the ForSaTum project funded by the EU-NRW Objective 2 program should be mentioned, which implemented prototypical scanners with analog and digital silicon photomultipliers (SiPMs).
- Experimental developments to increase the spatial resolution of PET - so far only applicable to small animal brains (rats) - have advanced to practical application; One example of this is the international collaboration between research institutes within the framework of the Crystal Clear Collaboration. This collaboration, hosted by CERN, to develop detectors for high-energy physics has developed a new generation of scanners, including ClearPET and ClearPEM, as part of technology transfer projects. Local resolutions of around <1.6 mm can be achieved.
- In the context of particle therapy , it is desirable to monitor the precise energy delivery to the patient. This is primarily intended to avoid incorrect irradiation. During the irradiation, nuclear fragmentation occurs in the tissue and as a result prompt particles are generated, including positron emitters. These can be measured with a suitable PET device used during the irradiation. The image obtained in this way provides information about the position of the positron emitter and thus also the nuclear fragmentation, which allows conclusions to be drawn about the location of the energy output.
Potential of technology
One of the limiting factors of today's PET is the low sensitivity of current systems. In the section on sensitivity it was described that the best PET devices achieved sensitivity values of almost ten counts per second and kilobecquerel in 2010 . This means that out of 1000 annihilations in the vicinity of the detector only ten are recorded, which corresponds to a yield of one percent. The ratio of the measured activity to the total patient activity is in the range of well below 1 ‰, because only those events are measured that are in the field of view of the detector. It can be assumed that faster real-time signal processing and an expanded field of view will be used in the future. This will further increase the sensitivity and the signal-to-noise ratio of the devices, which allows the use of weakly enriching and short-lived tracers and significantly reduces the examination time.
Such an expansion of the field of view can be achieved through the use of dedicated systems which are attached close to the organ to be examined. This is the case, for example, with positron emission mammography (PEM). The only currently commercially available positron emission mammograph, Naviscan Flex, thus achieves a normalized sensitivity of 0.15%. Competing designs and brain or prostate scanners under development are expected to perform similarly or better. Another possibility would be to expand the axial field of view of a whole body scanner. The main problem here is the large number of detectors to be used and, as a result, channels to be read out, which cannot be managed cost-effectively with current technology. Furthermore, the problem of the increasingly expected scatter coincidences would have to be solved. In February 2007, for example, Dario Crosetto was awarded a patent for such a technology, in which the axial field of view would be expanded to around 120 cm (instead of the 15 to 20 cm customary today). He also describes a new electronic detector which, if technically feasible, could overcome the problems described above.
In 2018, the University of California, Davis ( UCDavis ) presented a full-body scanner . In addition to a 20 to 40 times higher sensitivity (depending on the comparative scale) compared to conventional systems, it also offers a spatial resolution in the range of and below 3 mm, as well as the possibility of making dynamic PET recordings that show the distribution and accumulation of the tracer show all over the body. In addition, the device can still be used to obtain diagnostically useful images ten hours after the tracer has been injected. With the help of 500,000 detector elements, it covers a field of view 1.94 m in length.
Research institutions
In the German-speaking area one deals with a. in the following research institutions with the further development and limits of PET:
- RWTH Aachen University , Experimental Molecular Imaging, Teaching and Research Area Physics of Molecular Imaging Systems
- Helmholtz Center Dresden-Rossendorf
- university hospital Erlangen
- University Hospital Essen
- German Cancer Research Center Heidelberg
- research center Julich
- University Hospital Leipzig , Clinic and Polyclinic for Nuclear Medicine
- University Medical Center Schleswig-Holstein , Lübeck Campus, Clinic for Radiology and Nuclear Medicine
- Johannes Gutenberg University Mainz , Clinic for Nuclear Medicine, Institute for Nuclear Chemistry
- Clinic of the University of Munich ( LMU Munich ), Clinic and Polyclinic for Nuclear Medicine, Radiopharmaceutical Center RPC
- Klinikum rechts der Isar ( TU Munich ), nuclear medicine
- Westfälische Wilhelms-Universität Münster , nuclear medicine
- RheinAhrCampus Remagen , Department of Mathematics and Technology
- Ulm University Hospital, Clinic for Nuclear Medicine
- University Hospital Tübingen
- University Hospital Bern, Inselspital , University Clinic for Nuclear Medicine, Bern
- Max Planck Institute for Neurological Research
- University Hospital Cologne , Clinic and Polyclinic for Nuclear Medicine, Institute for Radiochemistry and Experimental Molecular Imaging (IREMB)
Similar procedures
literature
- O. Schober, W. Heindel: PET-CT. Georg Thieme Verlag, 2007, ISBN 3-13-143221-7 .
- J. Ruhlmann et al.: PET in oncology: basics and clinical application. Springer, 1998, ISBN 3-540-64632-9 .
- K. Wienhard among others: PET: Basics and applications of positron emission tomography. Springer, 1989, ISBN 0-387-19451-7 .
- EE Kim et al. (Eds.): Clinical Pet: Principles and Applications. Springer 2004, ISBN 0-387-40854-1 .
- Marcus Bauser, Lutz Lehmann: Positron Emission Tomography . In: Chemistry in Our Time . tape 46 , no. 2 , 2012, p. 80–99 , doi : 10.1002 / ciuz.201200564 .
- M. Vogel: Look into the body . In: Physics Journal . tape 15 , no. 1 , 2016, p. 42-43 . (Good, compact review article)
Web links
- PET - positron emission tomography: a look into the metabolism. Cancer information service of the German Cancer Research Center (DKFZ), Heidelberg. May 17, 2010. Retrieved September 4, 2014.
- PET - modern diagnostic method in the fight against cancer. German Society for Nuclear Medicine V. and Professional Association of German Nuclear Medicine e. V. (PDF; 369 kB)
- Possibilities and limits of PET tumor diagnostics
- Find a PET location nearby. German Society for Nuclear Medicine
- Nuclear Medicine Information - PET Topic Collection
Individual evidence
- ↑ Bernd J. Krause, Andreas K. Buck, Markus Schwaiger: Nuclear medicine oncology. ecomed Medicine, 2007, ISBN 978-3-609-76308-8 , p. 20.
- ↑ See technology of positron emission tomography, section Correction of the measurement data, subsection absorption correction
- ↑ L. Geworski: Requirements for quantification in emission tomography. Habilitation thesis, Humboldt University Berlin, 2003.
- ^ H. Herzog: Methods and applications of positron-based medical imaging . In: Radiation Physics and Chemistry . tape 76 , no. 2 , 2007, p. 337-342 , doi : 10.1016 / j.radphyschem.2006.03.063 .
- ↑ Bryce JB Nelson, John Wilson, Susan Richter, M. John M. Duke, Melinda Wuest: Taking cyclotron 68Ga production to the next level: Expeditious solid target production of 68Ga for preparation of radiotracers . In: Nuclear Medicine and Biology . tape 80-81 , January 1, 2020, ISSN 0969-8051 , p. 24–31 , doi : 10.1016 / j.nucmedbio.2020.01.005 ( sciencedirect.com [accessed May 29, 2020]).
- ^ Website of the Clinic for Nuclear Medicine at Ulm University
- ↑ Article in medscape.com
- ↑ Jens Cardinale, René Martin, Yvonne Remde et al .: Procedures for the GMP-Compliant Production and Quality Control of [18F] PSMA-1007: A Next Generation Radiofluorinated Tracer for the Detection of Prostate Cancer. In: MDPI.com. Pharmaceuticals 2017, 10 (4), 77, September 27, 2017, accessed November 22, 2018 .
- ↑ Schwenck, J. Rempp, H. Reischl, G. et al .: Comparison of 68Ga-labeled PSMA-11 and 11C-choline in the detection of prostate cancer metastases by PET / CT . In: European Journal of Nuclear Medicine and Molecular Imaging . Volume 44, No. 1 , January 2017, p. 92-101 .
- ↑ Amyvid now approved by European regulators. In: Dotmed.com , January 16, 2013.
- ↑ Gallium-68 peptide diagnostics. DKFZ
- ↑ guidelines. Website of the German Society for Nuclear Medicine, accessed on November 25, 2013
- ↑ Technical information. Website of the PET Center of the Ulm University Hospital
- ^ PET-CT Center Linz ( Memento from February 27, 2011 in the Internet Archive )
- ↑ Angela Aldin, Lisa Umlauff, Lise J Estcourt, Gary Collins, Karel GM Moons: Interim PET results for prognosis in adults with Hodgkin lymphoma: a systematic review and meta-analysis of prognostic factor studies . In: Cochrane Database of Systematic Reviews . January 13, 2020, doi : 10.1002 / 14651858.CD012643.pub3 ( wiley.com [accessed July 16, 2020]).
- ↑ a b c P. D. Shreve et al: Pitfalls in Oncologic Diagnosis with FDG PET Imaging: Physiologic and Benign Variants. In: Radiographics 19/1999, pp. 61-77. PMID 9925392
- ↑ Usefulness and limits of the SUV for tumor characterization and patient monitoring in FDG / PET (PDF; 4.3 MB)
- ↑ Radiation protection when using positron emission tomography / computer tomography (PET / CT); Statement by the Commission on Radiation Protection ( Memento of January 14, 2012 in the Internet Archive ) (PDF 850 kB).
- ↑ Announcement of the diagnostic reference values for radiological and nuclear medicine examinations from July 10, 2003. Federal Office for Radiation Protection (PDF; 84 kB)
- ↑ Peter Hien: Practical Pneumology . Springer DE, January 1, 2012, ISBN 978-3-642-10209-7 , p. 421.
- ^ Website of the Clinic for Nuclear Medicine at Ulm University .
- ↑ Hirsch, FW et al .: PET / MR in children. Initial clinical experience in pediatric oncology using an integrated PET / MR scanner. doi: 10.1007 / s00247-012-2570-4 .
- ↑ a b The positron emission tomography (PET) ( Memento of March 3, 2006 in the Internet Archive ) (PDF; 893 kB) Diagnoseklinik München, p. 7. (2006).
- ↑ C. Plathow, M. Walz, MP Lichy, P. Aschoff, C. Pfannenberg, H. Bock, SM Eschmann, CD Claussen, HP Schlemmer: Cost considerations for whole-body MRI and PET-CT in the context of oncological staging . In: The Radiologist . tape 48 , no. 4 , September 20, 2007, p. 384-396 , doi : 10.1007 / s00117-007-1547-z , PMID 17891370 .
- ↑ Guideline for methods of contracted medical care (PET for small cell lung cancer). Federal Joint Committee
- ↑ PET / computed tomography (CT) for malignant lymphoma. Resolution of the Federal Joint Committee on an amendment to the guidelines for hospital treatment methods (PDF; 240 kB).
- ↑ Decision of the evaluation committee according to § 87 Abs. 1 Satz 1 SGB V in its 369th meeting on December 15, 2015 to change the uniform evaluation standard (EBM) with effect from January 1, 2016.
- ↑ Quality assurance: Agreement on PET and PET / CT adapted , KBV. Retrieved February 14, 2018.
- ↑ Section 34.7 "Diagnostic positron emission tomography (PET), diagnostic positron emission tomography with computed tomography (PET / CT)" added to EBM. , KBV, January 1, 2016. Retrieved February 14, 2018.
- ↑ a b c d e f Sally F. Barrington, Michael N. Maisey, Richard L. Wahl: Atlas of Clinical Positron Emission Tomography. Second Edition, Hodder Arnold 2006, pp. 24, 25 ISBN 0-340-81693-7
- Jump up ↑ Igle J. de Jong, MD1,2, Jan Pruim, PhD2, Philip H. Elsinga, PhD2, Willem Vaalburg, PhD2, Han J. Mensink, PhD1, Department of Urology: Preoperative Staging of Pelvic Lymph Nodes in Prostate Cancer by 11C -Choline PET. 2 PET Center, Groningen University Hospital, Groningen, The Netherlands
- ^ The PSMA-PET / CT website of the Federal Association for Prostate Cancer Self-Help eV
- ^ A b Paul Shreve, David W. Townsend: Clinical PET-CT in Radiology Integrated Imaging in Oncology Springer Science + Business Media, LLC 2011 ISBN 978-0-387-48900-1 , Chapter 32: PET-CT of Bone Metastases, by James A. Scott and Edwin L. Palmer
- ↑ Michel M. Ter-Pogossian, Michael E. Phelps, Edward J. Hoffman, Nizar A. Mullani: A Positron-Emission Transaxial Tomograph for Nuclear Imaging (PETT) . In: Radiology . tape 114 , no. 1 , 1975, p. 89-98 , doi : 10.1148 / 114.1.89 .
- ↑ Michael E. Phelps, Edward J. Hoffman, Nizar A. Mullani, Michel M. Ter-Pogossian: Application of Annihilation Coincidence Detection to Transaxial Reconstruction Tomography . In: J Nucl Med . tape 16 , no. 3 , February 1, 1975, p. 210-224 ( abstract [accessed February 19, 2011]).
- ↑ Derek Lowe, Das Chemiebuch, Librero 2017, p. 450
- ↑ Epileptologie-bonn.de ( PDF )
- ^ A b Gordon L. Brownell: A history of positron imaging ( Memento of December 18, 2011 in the Internet Archive ) (PDF; 997 kB).
- ↑ Project approved in 1998: positron emission scanner. ( Memento of the original from December 2, 2013 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. Gebert-Rüf Foundation, accessed November 25, 2013.
- ↑ Ronald Nutt receives an honorary doctorate for his research on PET. DGN press release, 2008.
- ^ Michael Haas: PET Evens the Score in PET / CT. In: Imaging Technology News , July / August 2007.
- ↑ Gemini TF on the Philips Medical Systems website
- ^ Performance of Philips Gemini TF PET / CT Scanner with Special Consideration for Its Time-of-Flight Imaging Capabilities .
- ^ Siemens Medical website .
- ^ Discovery PET / CT 690 for Exploration. ( Memento of December 2, 2013 in the Internet Archive ) ITN, June 16, 2009.
- ↑ "9komma4" - a top class tomograph. In: Deutsche Welle online , April 29, 2009, accessed November 25, 2013.
- ^ Information service for science online
- ↑ Press release Auntminnie announcement .
- ↑ Press release Klinikum rechts der Isar and Siemens AG (PDF; 656 kB).
- ^ Dan Harvey: Simultaneous Acquisition - A PET Detector Inside an MRI Bore Makes It Feasible. ( April 22, 2008 memento on the Internet Archive ) Radiology Today 8 (3): February 16, 2007.
- ↑ Thorsten M. Buzug, Dietrich Holz, Jens Bongartz, Matthias Kohl-Bareis, Ulrich Hartmann, Simone Weber: MRI Based Attenuation Correction for Brain PET Images . In: Advances in Medical Engineering . tape 114 . Springer, Berlin / Heidelberg 2007, ISBN 978-3-540-68763-4 , pp. 93-97 , doi : 10.1007 / 978-3-540-68764-1_15 .
- ↑ Helmholtz-Zentrum Dresden-Rossendorf: Correction of head movements in PET ( Memento from February 24, 2013 in the Internet Archive )
- ↑ Motion-free PET: Compensating for patient respiration in whole-body PET / CT imaging ( Memento of the original from February 18, 2009 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. .
- ↑ Feng Qiao, Tinsu Pan, John Clark, Osama Mawlawi: Anatomy based PET image reconstruction for a motion influenced volume. Rice University, Houston, Texas; MD Anderson Cancer Center, Houston, Texas.
- ↑ Ralph A. Bundschuh, Axel Martinez-Moeller, Markus Essler, Maria-Jose Martinez, Stephan G. Nekolla, Sibylle I. Ziegler, Markus Schwaiger: Postacquisition Detection of Tumor Motion in the Lung and Upper Abdomen Using List-Mode PET Data: A feasibility study . In: J Nucl Med . tape 48 , no. 5 , April 1, 2007, pp. 758-763 , doi : 10.2967 / jnumed.106.035279 .
- ^ R. Brinks, M. Busch: Local Compensation for Respiratory Motion in List-mode PET. In: Th. Buzug, et al .: Advances in Medical Engineering. Springer Proceedings in Physics. 2007.
- ↑ Volkmar Schulz, Bjoern Weissler, Pierre Gebhardt, Torsten Solf, Christoph W. Lerche, Peter Fischer, Michael Ritzert, V. Mlotok, Claudio Piemonte, B. Goldschmidt, S. Vandenberghe, A. Salomon, T. Schaeffter, PK Marsden: SiPM based preclinical PET / MR insert for a human 3T MR: first imaging experiments . In: Nuclear Science Symposium and Medical Imaging Conference (NSS / MIC), IEEE . October 2011, p. 4467-4469 , doi : 10.1109 / NSSMIC.2011.6152496 .
- ↑ Jakob Wehner, Bjoern Weissler, Peter Dueppenbecker, Pierre Gebhardt, David Schug, Walter Ruetten, Fabian Kiessling, Volkmar Schulz: PET / MRI insert using digital SiPMs: Investigation of MR-compatibility . In: Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment . 734, Part B, January 11, 2014, p. 116-121 , doi : 10.1016 / j.nima.2013.08.077 .
- ↑ ClearPET offers improved insight into animal brains. In: CERN Courier. Volume 45, No. 6, July / August 2005, pp. 27, 28.
- ^ The Crystal Clear Collaboration. - from "High Energy Physics" to "Medical Imaging"
- ^ GATE - Geant4 Application for Emission Tomography .
- ↑ Weidong Luo, Edward Anashkin, Christopher G. Matthews: Performance Evaluation of a PEM Scanner Using the NEMA NU 4 - 2008 Small Animal PET Standards. In: IEEE Transactions on Nuclear Science. 57, 2010, pp. 94-103, doi: 10.1109 / TNS.2009.2036847 .
- ↑ Patent US7180074 : Method and apparatus for whole-body, three-dimensional, dynamic PET / CT examination. Published on February 20, 2007 , inventor: Dario B. Crosetto.
- ↑ D. B Crosetto: The 3D complete body screening (3D-CBS) features and implementation . In: Nuclear Science Symposium Conference Record, 2003 IEEE . tape 4 , 2004, ISBN 0-7803-8257-9 , pp. 2415-2419 , doi : 10.1109 / NSSMIC.2003.1352382 .
- ↑ UCLA Davis Explorer