Wing (insect)
The study of the insect wing is one of the central topics of entomology , the study of insects . Understanding its origin and its variety of forms is a major challenge for many biological sub-disciplines.
There are different systems for naming the veins, cells and fields of the wing. The evolution of the wings is largely unexplained and many questions are still unanswered with regard to the development during ontogenesis (individual development).
Already in the early Upper Carboniferous and thus more than 100 million years before the pterosaurs , the first airworthy terrestrial vertebrates that were able to fly with limbs converted into wings , the insects developed wings from protuberances of the skin . The development of the ability to fly made it possible to conquer new habitats and occupy numerous new ecological niches . As a result, there was extensive adaptive radiation , which means that flying insects are the most species-rich group of animals today. The success of the insects is due to the development of the wings.
| Fig. 0.1 Original veining and wing posture in a large dragonfly |
|

|

|
| Fig. 0.2 Hoverflies mating in the air |
Fig. 0.3 Hardened fore wings and foldable hind wings of the cockchafer |
evolution
Theories of origin
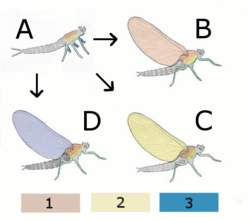
A Hypothetical wingless ancestor
B Paranotal theory
Hypothetical insect with wing from back (notum)
C Hypothetical insect with wing from side (pleurum)
D Epicoxal theory
Hypothetical insect with wing from appendix of legs
1 notum (back)
2 pleurum (Side)
3 exits (outer appendages of the legs)
From the Devonian (400 million years ago) only fossils of wingless urine insects are known, from the Carboniferous (320 million years ago) insects from more than 10 different genera with fully functional wings are known. There are no finds from the time in between showing transitional forms to a functioning wing.
The assumptions about the evolution of the wings are largely hypothetical. The theories as to from which part of the segments of the chest the wings developed can be divided into three groups (Fig. 1.1):
- Origin from lateral outgrowths of the insect,
- Derivation from outgrowths on the back of the insect, and
- Development from attachments of the legs of the insect (exit and endite theories).
Two main theories are discussed today.
- According to the epicoxal theory (also exit theory) ( Kukalová-Peck ), the wings were created from movable lateral attachments at the base of the legs . The ancestors of the insects therefore had articulated legs with external and internal appendages. From these legs, on the one hand, the split legs of the crabs developed, on the other hand, from the movable external appendix (called exit) of the uppermost leg link, the epicoxa, the wings of the insects. The epicoxal theory is mainly based on the fact that the wings of all insects are supplied with oxygen by a branch in the leg trachea, and on paleontological evidence.
- According to the Paranotal theory (first Müller, F., 1873), the wings were formed from rigid outgrowths of the back plates ( notum ) of the chest. This is supported by the fact that during the ontogenesis of insects with incomplete development, the wing systems are rigidly connected to this part of the body until the last moult.
Regardless of whether the wings arose from appendages of the legs or protuberances of the chest, the question remains unanswered as to why these wing precursors have evolved into functional wings. Reasons must be given as to why it meant a selection advantage that the initial wing stubs enlarged so that the function change from the flightless extension to the flightable organ was possible. There are several plausible hypotheses for this, which are summarized here.
- The body excesses were used for advertising. Larger wings could lead to a higher chance of reproduction.
- The wing precursors had a direct gill function or indirectly provided a better oxygen supply by ventilating the water. Their original mobility led to their wing enlargement via rowing function and flight-like rowing on the water surface (surface-skimming), coupled with the development of muscles and flexibility.
- The preforms of the wings had a protective function for the gills. Their size improved the protection of the gills and was therefore positively selected.
- The body excesses offered aerodynamic advantages. They made it possible to jump higher or further when hunting prey or when fleeing, or they brought a smaller risk of injury when falling from a great height. Even a higher probability of landing on your feet when falling would mean a selection advantage. Flying could thus be developed through gliding.
- The preforms of the wings were used for heat regulation. As cold-blooded animals, insects are lethargic in cool surroundings. It is advantageous for them if they reach the temperature necessary for mobility earlier than the other cold-blooded animals when they warm up. The preforms of the wings, which are exposed to the sun, are said to have been crisscrossed with a dense network of veins. The heat absorbed by solar radiation could have been transported into the body so efficiently. Heat stored in the veins of the wings would also make it possible to extend the period of activity when cooling down. The easiest way to enlarge the body surface and at the same time keep the volume to be heated small are outgrowths of the body surface in the form of thin plates, which ideally unfold when heated, are held close to the body when cooled and are rotated and inclined when unfolded that they are at a good angle to the sunlight.
To weigh up these hypotheses, at least something must be known about the insect group in question, in which the development of the wings began. Depending on whether it was aquatic or land animals, one or the other hypothesis becomes more plausible, depending on the size, the aerodynamic considerations have more weight, depending on the way of life and reproductive biology, the hypotheses must be assessed differently.
| Fig. 1.2: Evolution of the options for keeping the wings at rest |
||
|---|---|---|
| Wings cannot be folded back (recent archaeoptera) |
laterally spread out (large dragonflies) | |
lying next to each other over the back (dragonflies, mayflies) |
||
| Foldable (Neoptera) |
||
| Wings not foldable (e.g. stone flies) | ||
| Foldable | Longitudinal folding (e.g. front wing of the wasps, Fig. 1.3) |
|
| Transverse folding (e.g. hind wings of beetles, Fig. 1.4) |
||
| Fan folds (e.g. hind wings of the earwigs, Fig. 1.5) |
||
Fossil finds of the genera Stenodictya from the Carboniferous and Lemmatophora from the Permian (270 million years ago) show, in addition to simple wings on the middle and rear breast sections, wing stub-like protruding outgrowths on the first breast segment. These stubs, which used to be seen as a reduced third pair of wings, now find a far more conclusive explanation with several of the above-mentioned hypotheses (picture on web links). In all cases, a rapid increase in wing size and mobility can be expected. Sufficiently large outgrowths would enable the possibility of gliding flight and drifting through wind and thus initiate the change in function to locomotion organs.
In order to reconstruct the origin of the insect wings, scientists from the Japanese research group led by Shigeo Hayashi recently studied the expression of three regulatory genes, which are crucial for wing formation, in representatives of two groups of insects, namely in winged but very primitive mayflies (Ephemeroptera) and in silverfish, i.e. wingless urine insects of the order Zygentoma.
Various body appendages from these primitive groups were previously considered to be the precursors of the wings. The results can be interpreted in such a way that two separate development-controlling modules are present in the wingless urine insects. One acts on the sides of the body (lateral) and controls the formation of the upper, rod-shaped branch of the branched limbs. The other acts on the seam between the side of the body and the back and controls the formation of flat, shield-shaped outgrowths there.
The authors propose a model according to which the upper (dorsal) branches of the limbs were included in the area of the seam between the side of the body and the back, and assumed the shape of the flat outgrowths found there. This was done by simply integrating the two previously separate control modules, so that afterwards both the flat, two-layer design of the wings became available, as well as the muscles (belonging to the limbs) that could perform movements. This enabled a rapid evolution of the insect's wings. The present model shows that even major changes to the construction plan, which have an enormous impact on the ecology and further tribal history of an animal group, can be explained at the level of development control by relatively few change steps.
The discovery of the fossil larvae of the Coxoplectoptera in 2011 provided new clues to clarify the evolutionary origin of the insect wings, which led to a hypothesis similar to that of the Hayashi working group. So far, the two theories, Paranotal theory and Epicoxal theory, were considered incompatible alternatives, each of which was supported by different evidence from the fossil record, comparative morphology, developmental biology and genetics. The proof that leg genes are expressed in the ontogenesis of the larval wing anlage was considered convincing evidence for the epicoxal theory, which derives the insect wing from transformed, movable branches (exits) of the split legs. The larvae of the Coxoplectoptera show, however, that the abdominal gills of the mayflies and their ancestors, which are regarded as organs serially corresponding to the wings, arise within the dorsal shields. This is not recognizable in modern mayflies, since the back and belly shields in the larvae's abdomen are always fused into rings, and there are no indications in the embryo either. If larval gills and wings are corresponding ( serially homologous ) structures and thus have the same evolutionary origin, the finding of the Coxoplectoptera larvae means that the wings are also of Tergal origin, as the classical Paranotal theory said. Staniczek, Bechly & Godunko (2011) therefore proposed a new hypothesis that could reconcile the new paleontological findings with the results of developmental genetics. According to this, wings were initially formed as rigid outgrowths of the back shields (Paranota), and only later in evolution would these outgrowths have become mobile through the secondary "recruitment" of legs.
Paleontological Findings
Although clear intermediate stages of winged and wingless insects have not yet been found, which is repeatedly emphasized by opponents of the theory of evolution, a development within winged insects can be proven through fossils. The oldest winged insect found so far is about 324 million years old, named after its place of discovery in the Saxon / Saxony-Anhalt mining area Bitterfeld / Delitzsch Delitzschala bitterfeldensis (picture on web links). There is also a site for fossil insects in Germany near Hagen-Vorhalle ( Westphalia ). Sites are distributed all over the world, a reference to a series of eight world maps from eight geological ages with the location of finds from these times can be found in the web links section.
| Fig. 1.3: Longitudinal folding using the example of the wasps (Vespidae) | |
|---|---|

|
In the picture above , the main fold line of the fore wing can be seen as a light horizontal line about halfway up . The part of the wing that is behind this line is folded back and down. The narrow strip on the leading edge of the wing in front of the first strong vein is folded down towards the front. |

|
In the rest position, the vein forms the resistant outer edge of the wing, which protects the sides of the abdomen like a kind of shock absorber. The rear wing is largely enveloped by the fore wing. |
In view of the fundamental difficulties involved in fossilizing such fragile creatures in ancient rock strata, however, it is to be expected that many finds leave room for interpretation. Another fundamental difficulty is that a fossil-based innovation must have been developed earlier. The finds are supplemented by temporal estimates with the molecular clock . According to these, the first flying insects appeared in the Devonian approximately 390 million years ago. The division into Palaeoptera and Neoptera is suspected in the middle Devonian.
The Neoptera (new winged birds) include all current insect orders with wings except for the mayflies and dragonflies as well as the insect orders in which the wings have receded. They have a uniform mechanism to fold their wings backwards, which is why a common ancestor is assumed ( monophyletic origin ). A Radiation of Neoptera took place probably in the upper Carboniferous , as in the Perm already the majority of today's systems can be seen. Only highly specialized orders of insects appeared later. For example, fleas with receding wings can only be detected in the lower Cretaceous period .
In the case of the Palaeoptera (adult-winged birds, according to other authors Palaeopteroidea) with about seven orders, five of which are extinct, the systematic relationships are still unclear. With regard to the wings, three groups are of interest.
| Fig. 1.4: Cross folds in the rose beetle | |
|---|---|

|
Hind wings spread. It is divided into five fields by folding lines, each of which is closed at the back by an arch. |

|
The same wing folded in half. The two joints for the transverse fold form an obtuse angle. To the right of this, the wing is already folded in three layers. At a higher resolution, the third arch of the wing edge is visible under the first and second. To the left of this the fifth arch appears under the fourth. |

|
The same wing fully folded. The five fields are on top of each other. (The front wing has been removed) |
- In one order of the Palaeoptera, the extinct Diaphanopter (oide) a , there is an ancient type of folding the wings backwards. This shows that the possibility of folding wings when at rest was developed twice in carbon independently of one another ( polyphyletic ). The idea suggests that this folding meant a selection advantage or, in other words, that non-folding wings were disadvantageous for certain habitats and forms.
- Apart from the mayflies, the dragonflies are the only order of today's winged insects that are not classified as Neoptera but as Palaeoptera and whose wings cannot be folded back when at rest. The ancient dragonfly Meganeuropsis permiana with a wingspan of 71 cm from the Permian is one of its ancestors . There are also fossils of paleozoic dragonfly larvae whose wings are so well developed that one can assume that the larvae can fly . Even in the earliest known larval stages, other paleozoic flying insects had fully articulated wing sheaths, which are often spread out laterally, in contrast to the rigid and well-growing wing sheaths of modern flying insect larvae. The wing sheaths of these Paleozoic flying insect larvae were clearly curved in early larval stages, and in later larval stages, in addition to increasing in size, they also became straighter and more imaginal-like.
- In today's representatives of the mayflies, the hind wings are significantly smaller than the forewings. In fossil finds one finds forms in which the wings are still the same size. In addition, today's mayflies, which are among the most original flying insects, are the only flying insects with the interesting phenomenon that in addition to the actual imago , the last "larval stage" (subimago), which is only a transitional form, is still able to fly and thus still another last moult in airworthy condition. It could therefore be that limiting the ability to fly to the reproductive adult stage, as is found today in all other flying insects, had a selection advantage.
In contrast to the folding mechanism of the wings in the Diaphanoptera, the folding type of the Neoptera enabled certain wing veins to move relative to one another in such a way that the wings can be folded lengthways when they are at rest. As a rule, it only refers to the hind wings, only in the hymenoptera also to the forewings (Fig. 1.3). It can be proven that folds must follow certain fundamental principles. That is why these are also implemented in all orders in which wing folding occurs. The intuitive conclusion that the similar construction can be traced back to a common ancestor is therefore not justified. Rather, the paleontological findings suggest that the folding mechanisms within the orders were invented independently of one another. The longitudinal fold is older and occurs in eight recent orders. The later occurring transverse fold (Fig. 1.4) can be found in four recent orders, where it was probably developed independently of one another. The third type of folding, which works according to a fan, occurs in six recent orders and is also not due to any common ancestor (Fig. 1.5).
| Fig. 1.5: Fan folds in an earwig | |
|---|---|
| In the picture opposite, the front and rear wings are at rest on the left . The fore wing largely covers the hind wing . Only the joint protrudes from this in the form of a quarter circle with a central white spot under the forewing. On the right side of the picture, the front wing is opened to the right (blue arrow). In perspective, it appears narrower than it is. The hind wing is still completely folded. |
|

|
In the picture on the left , the front wing is open to the left (blue arrow). On the right side, the front wing has been removed. The hind wing is half open. At a higher resolution, the multiple folds, like a fan, can be clearly seen parallel to lines b and c . The arrow e points to the point at which the closed fan is folded again by 180 ° . Finally, the double stack of wing panels is pushed under the large wing panel that is parallel to line a. |
The scope of fossil finds is still far from sufficient to answer the open questions regarding the development of the insect wing with a closed and generally accepted theory. However, they provide material for interesting speculation. An important hypothesis states that the contradicting selection pressure on the wings, which are bulky for the larvae on the one hand, and the size of the wings, which is important for flight ability, was answered in two ways. In one group, wing development was reduced to the last stage before the imago, which ultimately led to the development of complete metamorphosis . As an alternative, the wings were aerodynamically placed backwards during the larval stages and lost their original mobility, which they only regain during the last moult , as can be seen today with the incomplete transformation. With the wings one could explain the division of insects into holometabols and hemimetabols.
A summary of the fossil history of the individual insect groups can be found in Rasnitsyn and Quicke.
Wing construction
General
Flying insects have four wings in the basic plan. One pair, the forewings, sit on the middle ( mesothorax ), another pair, the hind wings, on the posterior segment of the thorax ( metathorax ). Wings are never attached to the first (front) breast segment, the prothorax . In evolutionarily original insect orders, the fore and hind wings are very similar in shape and have comparable veining, but often one pair of wings is larger than the other, usually the hind wings are larger. The front pair of wings can be transformed into deck wings or hemielytres protecting the hind wings and then only contribute little to the lift in flight (see the section on the protective function below). In some groups, a pair of wings, or even both, are secondary to regressions. There are many other modifications, which are mostly of importance for the systematic classification and which are discussed below in the form of a table. However, great differences can also occur within an order, sometimes even within a species, for example between the sexes. For example, with some locust species all transitions from normal quadrupeds to wingless occur, with fireflies only the males have wings, etc. The following paragraphs therefore only describe the basic wing structure.
The skin wings of the insects consist of a double layer of cuticle that merges directly into the cuticle of the thorax . This double layer can differ in thickness (more chitin ) and hardness (more sclerotin ) independently of one another . The actual membrane is very thin (only about a micrometer), and in some areas, often in the form of a line, not very hardened, which on the one hand enables the mobility of the synovial membrane when it is turned into the thorax, and on the other hand aerodynamically important deformations during the flight and folding Resting position allowed. However, there are also membrane areas in the form of thin, hardened plates that can be bent but not warped. The stiffening of the membranous sections and the plates against each other takes place through the sclerotized wing veins. They are up to 100 micrometers thick, but can be bendable in sections in predefined directions and to a limited extent. They are mostly hollow and after molting are inflated with hemolymph , causing the wings to unfold. They then harden in the air and are usually recognizable by their darker pigmentation . The nerves and trachea of the wings, if any, run in the veins. Muscles do not exist within the skin wings.
| Fig. 2.1: Movement of the insect wings with indirect flight muscles Schematic cross section through a breast segment with wings |
|---|
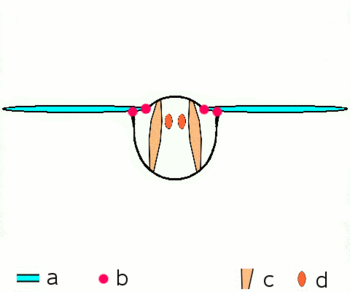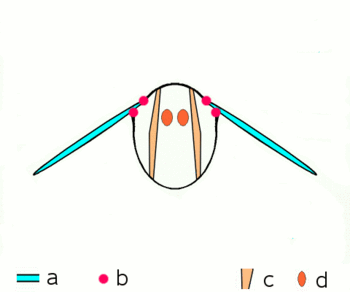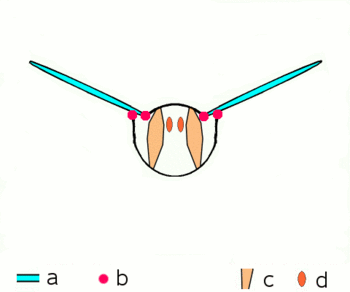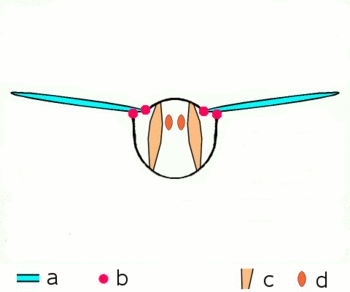
|
| a Wings b Joints c Dorso- ventral and d longitudinal muscles The strong dorso-ventral musculature (c), which runs from top to bottom, deforms the elastic chest ring with its contraction, while the wings move upwards. Then the dorsoventral muscles relax, the chest ring snaps back into its natural position, supported by the contraction of the longitudinal muscles (d). The wings move downwards. For more information on the joints, see below |
The bracing of the wings by the tubular veins allow rotations and distortions only to a limited extent and in certain directions and angles. At the base of the wing, the veins form a joint system with the cuticle of the thorax in the form of various shoulder sclerites, which guarantees mobility during flight and enables the wings to rest. The phylogenetically older flight movements are caused by levers by alternating contractions of the chest muscles in the vertical direction and in the direction of the body axis (Fig. 2.1). When the chest muscles that run from the back to the abdomen contract, the wing is lifted; when the muscles that pull the chest through from front to back contract, the previous flattening of the chest is lifted and the wing is lowered. Further muscle groups, which are attached to the underside of the base of the wings, regulate the wing position and wing shape during the wing beat and allow the Neoptera to move in the shape of a figure eight.
Venation and cells
A characteristic feature of the wings is their veining. On the basis of these, orders and families and even individual genera and species can be distinguished.
There are various naming systems to describe the vein. They designate the veins and the wing areas created by them. The naming systems have two z. T. contradicting goals. On the one hand, a naming system would be desirable in which the original vein system is reflected, so that the vein types occurring today can be derived as specializations. The derivation of today's vein types from an original form would have a high systematic importance, but has not yet been clarified. On the other hand, there are practically oriented naming systems with which the range of vein variation occurring within a systematic group can be described simply and clearly. In some groups of insects there are so-called false veins , which only have the appearance but not the structure of the vein and therefore cannot be included in an original system.
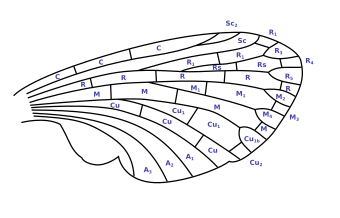
One of the first and probably also the most widespread model of a systematic naming of the vein is the Comstock-Needham system created in 1898 (Fig. 2.2, 2.3). It was an important means of showing the homology of insect wings. The system provides the following names for the six large longitudinal veins in the wing, starting at the leading edge:
- Costa (C) ,
- Subcosta (Sc),
- Radius (R),
- Median (M),
- Cubitus (Cu),
- Anal veins (1A, 2A, ..).
If the veins split, their names are also indexed with numbers.
The differences in the naming of the veins in other naming systems often relate only to the rear part of the wings. For example, Snodgrass calls the first anal vein postcubitus (PCu) instead of A1 and then starts numbering them as vannal veins from the second anal vein . Various authors also consider the vein (s) in the rearmost wing field, the jugal field (see below), to be primary and supplement the system shown above with the juga (J) towards the rear . Jugaladers do not appear in all orders.
| Veining in different flies | |
|---|---|
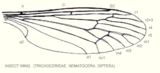
|

|
| Fig.2.4 Venation of the Trichoceridae |
Fig. 2.5 Veining of the Lonchopteridae |

|

|
| Fig. 2.6 Veining of the Phoridae |
Fig. 2.7 Reduced venation in Ichneumonidae |

|
|
| Fig. 2.8 Strongly reduced venation in Braconidae |
Fig. 2.9 Extremely reduced venation in Chalcidoidea |
The areas delimited by the individual veins, the so-called cells, are also named. A cell is said to be closed when it is delimited on all sides by veins, and open when one side extends to the edge of the wing. The name of the cell after Comstock-Needham is derived from the vein in front of it. For example, the cell between Sc 2 and R 1 is referred to as Sc 2 . If the veins are split up, the various systems naturally provide for further differentiated names. The designation for the Comstock-Needham system is shown in Fig. 2.2.
In order to be able to better describe the peculiarities of the wing construction of certain orders, further naming systems were created. For example, there are five other naming systems for dragonflies that are used in parallel to that of Comstock-Needham.
In phylogenetically more modern forms, one can see an increasing differentiation of the vein structure and a reduction in the number of veins and thus the cells. However, reduced veining does not necessarily mean that the insect group is phylogenetically younger, since the number of veins present also depends on the size of the insect. Figures 2.4, 2.5 and 2.6 show the veining of different dipteras . Almost all of the longitudinal veins are present in the Trichoceridae , which is almost one centimeter in size , but there are only two anal veins and the transverse veins are greatly reduced. The on average smaller longopterids (approx. Four millimeters) have fewer veins, and in the one to two millimeter large Phoridae the veining is even more reduced. The same phenomenon can be observed in the Hymenopteran wings in Figures 2.7, 2.8 and 2.9. The ichneumonids only have four radial veins, with the smaller braconids the veining is significantly reduced and with the chalcidoidea , to which the smallest winged insects belong, the veining is extremely reduced.
Fields
Unfortunately, the names of the various wing areas are also not uniform. In the Comstock-Needham tradition, the wing is divided into fields bounded by the longitudinal veins. The fields are generally named after the vein that limits the field to the front. The costal field is behind the costa, the subcostal field behind the subcosta, the medial field behind the media. The praecostal field, on the other hand, lies in front of the costa, the anal field is bounded in front not by a vein but by the anal fold, and the pterostigma is also often referred to as the wing field, although it is part of the costal field. Here, too, the common names primarily serve to describe characteristics within an insect group. In the simplest case, e.g. B. with the phasmids, one differentiates only a costal and an anal field. In connection with the question of the evolution of insect wings, however, the folding lines (along which the wings are folded in the rest position) and the flexion lines (along which the wings bend during flight) have become important for the limitation of the wing fields (Wooton 1979, Fig. 2.10). The most important fold lines are the jugal fold (plica jugalis, behind the third anal fold) and the claval fold (plica clavalis, lies against the postcubitus). They divide the wing into three fields. In front of the claval fold is the remigium, which corresponds to the costal field. Behind between the claval and jugal folds lies the clavus, and behind the jugal folds the jugum. Occasionally one or more fold lines, the vannal folds, can be found between the jugal and claval folds of the hind wings. In this case, the area between the claval and jugal folds, in which the vannal folds lie, is not referred to as the clavus, but as the vannus, so that the fields Remigium, Vannus and Jugum result.
Joints
All longitudinal arteries converge at the base of the wing, where they are connected to the sclerites of the back either directly or by means of syndeses . The sclerites are also called axillaries. The following names have been used for the individual sclerites. The sclerite adjoining the costa is called humeral sclerite, followed by pterals 1 to 3, which in this order belong to subcosta, radius and anal. In some species there is also a pteral 4 that is not connected to any vein . These sclerites together form the secondary wing joint. The primary or pleural wing joint is formed on the lower wing surface by the Pteral 2 and the fulcrum which are connected by a membrane. The pteral 3 is responsible that the wings can be put on.
Wing construction and systematics
Even Aristotle named groups of insects after the construction of their wings. Today almost all insect orders are formed with the ending -ptera ( Greek πτερόν = wing, in insects in the sense of membranous wing). To simplify matters, one can say that the structure of the wings defines the insect orders, their veining the families. In the case of higher systematic units, -oidea ( εἶδος type, -like) is often added, resulting in the ending -pteroidea. In addition, groups of flying insects, in which there is no closer family relationship or this is controversial, are occupied with words in which the word part -ptera occurs. The first part of the word indicates a peculiarity of the wings that characterize the group. The following table is sorted alphabetically by scientific terms so that it can be used as a glossary.
| Scientific name | linguistic root | Translation of the scientific name | German name | Remarks |
| Anisoptera | ἀνισο - aniso- unequal | Uneven winged | Dragonflies | Subordination, in contrast to the small dragonflies, hind and front wings are of different sizes. Note 7 |
|
Aptera apter |
ἀ - without | Wingless wingless |
--- wingless |
Order at Linnaeus , now dissolved, insects can be primarily or secondarily wingless, remark 1 |
| Apterygota |
πτερύγιον pterygion (small) wing ἀ without |
Wingless | Urine insects | (plesiomorphic) subclass, summary of all primarily wingless insect orders, cf. Note 1 |
| Coleoptera | κολεός koleos leather sheath into which the sword was put (sword scabbard) | Shrouded-wing | Beetle | Order, forewings form a protective cover. Note 5 |
| Dermaptera | δέρμα derma skin, leather | Leather wingers | Catchy tunes | Order, fore wing shortened and leathery, remark 5 |
| Diaphanopteroidea | διαφανής diaphanēs transparent, translucent | with transparent wings | --- | Extinct superordinate order |
| Dictyoptera | δίκτυον diktyon network | Flying insects with reticulate veins | Cockroaches, termites, fishing horrors | Order, system unclear |
| Diptera | δύο dyo two | Two-winged | Flies, gnats | Order, only one membranous pair of wings, remark 3 |
| Embioptera |
ἐν - en- inside βίος bios life |
Inner flying insects | Tares spinner | Order; spin tubes in which they live; only male winged |
| Endopterygota |
ἐντός entos inside πτερύγιον (small) wing |
Inner wing | --- | The wings develop inside the body; Pterygota with complete metamorphosis; synonymous with holometabola |
| Ephemeroptera | ἐφήμερος ephēmeros over (one) day (long) | Mayfly | Mayflies | Order, also winged last larval stage; this and Adult very short lived |
| Exopterygota | ἔξω exo outside | Outdoor flying insects | --- | Wings develop on the outside of the body; Pterygota with incomplete metamorphosis, synonymous with hemimetabola |
| Hemiptera | ἡμι - hēmi- half- | Half-winged | Schnabelkerfe (bugs, cicadas, aphids, ...) | Remark 2, remark 5 |
| Heteroptera | ἑτερο- hetero- different | Miscarriage | Bed bugs | Fore and hind wings are different, note 2, note 5 |
| Homoptera | ὅμο- homosexual at the same, similar- | Equal winged | Cicadas, aphids ... | The fore and hind wings are not different as in Heteroptera; Remark 2, remark 5 |
| Hymenoptera | ὑμένιον hymenion (thin) membrane | (Thin) hymenoptera | Hymenoptera (bees, ants, ...) | Order, remark 4 |
| Isoptera | ἶσον īson the same | Equal winged | Termites | Fore and hind wings are the same |
| Lepidoptera | λεπίς lepis , genitive: λεπίδος lepidos scales | Scaly wingers | Butterflies | All right, the wings have scales. Note 8 |
| Lonchopteridae | λόγχη lance (nspitze) | Lance flies | Lance flies | Diptera family, wing has the shape of a lance tip |
| Mecoptera | μῆκος mēkos length | Long-winged | Beak flies, beak flies (scorpion flies ...) |
order |
| Megaloptera | μεγαλο- large- | Large wing | Mud flies | Order or subordination, remark 11 |
| Neuroptera | νεῦρον neuron nerve, vein | Bloodwings | Reticulated winged | Order, wings strongly veined like a network |
| Neoptera | νέος neos new / young | New wingers | --- | includes all currently living orders of flying insects except mayflies and dragonflies |
| Oligoneoptera |
ὀλίγον oligon little νέος neos new |
Neoptera with few veins | --- | Synonym for holometabola, v. a. popular with Russian taxonomists. |
| Orthoptera | ὀρθο ortho - straight, right | Right winged | Locusts, crickets ... | The system is controversial, wings run straight back |
| Palaeodictyoptera | παλαιός palaios old δίκτυον diktyon network |
Alt-Netzflügler | --- | extinct order |
| Palaeoptera | παλαιόν palaion old | Adult winged | --- | System not clarified, mainly extinct orders, recent orders without a mechanism for folding the wings backwards |
| Paraneoptera | παρα- instead of, for νέος new |
Neoptera with false wings (hemielytres, paraelytres, ...) | Bugs, earwigs ... | Division of the Neoptera, systematics unclear |
| Phthiraptera | φθείρ phtheir louse ἀ without |
Animal lice | Animal lice | Order wingless ectoparasites , note 10 |
| Plecoptera | πλέκειν plekein fold | Folding wing | Stoneflies | Order, wings wrapped around the body at rest. |
| Polyneoptera | πολύς polys a lot νέος neos new |
Neoptera with many veins | --- | Division of the Neoptera, systematics unclear |
| Psocoptera | ψώχω psocho rub | Flying insects that gnaw | Dust lice | Order, fore and hind wings coupled at rest and in flight, with gnawing mouthparts |
| Pterostigma | στίγμα stigma stain, mark, wound | Wing spot | Wing paint | opaque or pigmented spot near the 1st wing vein (costa) in dragonflies, dust lice and hymenoptera |
| Pterothorax | θώραξ thōrāx chest | "Wing breast" | --- | All of the breast sections on which wings are attached, i.e. the whole breast except the 1st segment |
| Pterygota | πτερύγιον pterygion (small) wing | Wing insects | "Flying insects" | Subclass, as opposed to Apterygota, includes winged and secondarily wingless orders, note 1 |
| Raphidioptera | ῥαφίς needle | Coniferous | Camel neck flies | Order or subordination, remark 11 |
| Siphonaptera | σίφων siphōn suction tube, trunk ἀ without |
Trunk-wingless | Fleas | Order, secondary wingless (remark 1), with proboscis |
| Strepsiptera | στρέψις strepsis rotation | Rotary wing aircraft | Fan wing | Order, the forewings, which are transformed into holders, rotate when the preparation dries, remark 3 |
| Thysanoptera | θύσανοι thysanoi fringes | Fringed winged | Fringed-winged, thrips | Order, remark 6 |
| Trichoptera | τρίχωμα trichōma hair | Hair wingers | Caddis flies | Okay, the wings have hair |
| Zoraptera | ζωρός zōros strong, strong | Strong winged | Ground lice | Order, remark 9 |
| Zygoptera | ζεῦγος zeugos couple | Pair winged | Dragonflies | Subordination, in contrast to the large dragonflies, the front and rear wings are put together in pairs in the rest position. Note 7 |

Remarks
- An insect is primarily called wingless if its ancestors split off from the trunk of the other insects before the wings began to evolve. Secondary wingless, on the other hand, is an insect that has winged ancestors.
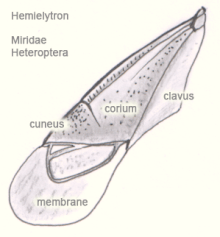
- The Heteroptera are either seen as an order, then the Hemiptera are a superordinate order, or the Hemiptera are seen as an order, then the Heteroptera are subordinate. Likewise, the Isoptera are viewed as an order, as a subordination to the Blattodea or (together with the Blattodea) as a subordination to the Dictyoptera. The word hemiptera refers to the fact that the fore wings are membranous only in the back. The anterior sclerotized part is divided into corium and clavus by a clavel fissure (Fig. 3.1). The hind wings are folded under the forewings when at rest. With the Homoptera, the wings are set up like a roof at rest, with the Heteroptera they lie flat on the abdomen.
- In the Diptera and Strepsiptera , a pair of wings is transformed into so-called swinging bulbs (holders), short, small outgrowths that are thickened like a bulb at the end. In the Strepsiptera the front wings are holders and the rear wings are fully developed. In the Diptera the hind wings are holders (Fig. 3.2). Since these no longer look like wings, only the two fore wings remain, hence the name Diptera, two-winged. The holders swing in push-pull with the front wings and act as a balance organ, with which every change of direction can be perceived. In some groups, the sole purpose of moving the brackets is to preheat the muscles before the start.
- Hymenoptera typically have four wings. They are all skinned. In some families, the fore wings are also folded lengthways when at rest, the rear ones being smaller and barely recognizable under the fore wings when at rest (Fig. 1.3). With a row of small hooks on the leading edge of the hind wings (hamuli), these are coupled to the forewings. The venation is often greatly reduced and forms a few large cells, which is characteristic of the group (Figs. 2.4 to 2.6). A darker pterostigma is often found at the tip of the fore wings. A separate system is often used to name the veins (Herbert H. Ross 1936, Weblinks).
- Beetles ( Coleoptera ), cockroaches ( Blattodea ), earwigs ( Dermaptera , Fig. 1.5) and Schnabelkerfe ( Hemiptera ) are the four recent orders of insects that, in connection with the transformation of the forewings as cover wings with protective function, can also fold the hind wings across them to be able to hide under the forewings. It is not a question of separate developments of the fore and hind wings, but the two developments go hand in hand.
When the insect is not flying, the cover wings lie partially or completely protectively over the hind wings and abdomen. In the case of heavily reshaped forewings (beetles, earwigs), these are only splayed upwards at an angle during flight and support stability and dynamic lift . Then they do not carry out any flight movements and at most vibrate. After the flight, they are placed over the abdomen. In the case of the beetles, their insides touch and are folded together in the so-called seam ( sutura ) parallel to the longitudinal axis of the body, in the other groups they partially overlap.
Since the skin wings are larger than the cover wings, they are folded together longitudinally and transversely along the folding lines provided so that they can be pushed under the forewings. In the simplest case (scraping) there are only two additional folding lines running across, so that part of the wing can be folded in like with origami . In beetles, an additional joint system has developed in the middle of the wing. The wing musculature is used for tensioning, which, via the shape of the veins and a correspondingly complex system of snap and locking joints, causes the wings to lock in the opened position. These joints are designed in such a way that the wings remain open despite the forces that act on the joints during flight movements (Fig. 1.4). To fold the wings after the flight, the front wings are partially closed and the rear wings are then pushed underneath with the help of the legs and / or the abdomen. - The wings of the Thysanoptera (Fig. 3.3) are extremely specialized. If there are wings, they are 1 to 1.2 millimeters long and have around 150 to 200 of the eponymous fringes, which are so close together that they form 20 to 45% of the wing surface. The diameter of the fringes is one to two micrometers. The front and rear wings of the Thysanoptera are linked by a connection at the base of the wing during flight.
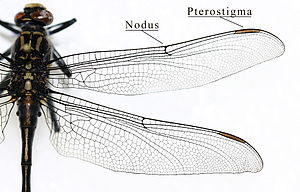
- Dragonflies ( Odonata ): The large fore and hind wings (up to 19 centimeters long and 18 millimeters wide) are approximately the same size in the small dragonflies and are usually placed upwards at rest, in the large dragonflies the hind wings are significantly wider than the forewings and the Wings remain spread out sideways at rest. The complex veining (Fig. 0.1) corresponds to the Comstock-Needham naming system. In contrast to the Neoptera, the wings cannot be folded back and have no folding lines. In addition, in contrast to almost all other flying insects, the flight muscles of dragonflies attach directly to the wings. The wings are stabilized by a series of longitudinal veins, between which the flight surface is not flat, but alternately inclined slightly up and down (Fig. 3.4). In the center of the wing is the nodus (knot), a transverse chitin thickening that connects the veins and prevents them from kinking during flight. At the front area of the wing tip, most species have an enlarged and darkly colored wing field, which is called a pterostigma and can be used as a trimming tank by filling it with hemolymph . The type of veining is used for systematic classification.
-
Lepidoptera (butterflies) The fore and hind wings are suspended individually, but in flight in some groups they are linked to one another by special mechanisms using a hook, the frenulum . The veins lose their function after drying out. The wings are covered with scales on the top and bottom (Fig. 3.5). The scales are flattened, species-specific hairs that lie on the wings like roof tiles and thus cover the wing veins. The individual scales are always only one color. Their shape varies greatly. The most common form is the shield-shaped with three to five points and a narrow stem anchored in a recess at the end. Others are lance-shaped or circular. The scales are not necessary for flying. In the case of the glass winged birds (Sesiidae), large areas of the wings are initially still loosely scaled, but on the first flight they become transparent and crystal clear due to the loss of the scales.
A separate naming system for the veins is used, which divides the wing into regions. The wings of a butterfly are described as an example: The regions run from the base of the wing to the tip, with each of the fore and hind wings being divided into four regions. The wires are numbered from 1 to 12 on the fore wings. The 1 at the back runs parallel to the inner edge. There are only 9 (rarely 10) continuous veins on the hind wings. The areas that are bounded by the wing veins are called cells or central or discoidal cells. You can find more information about the wings in the article Wings (butterfly) . - The Zoraptera are a species-poor order of very small mostly wingless insects, mostly warmer areas. They have no representatives in Europe.
- The Phthiraptera are small, dorsoventrally compressed, secondary wingless (see Note 1) ectoparasites on birds and mammals.
- The Raphidioptera have four identical, primitively veined wings. They belong to the Neuroptera in the broader sense and are also listed as a subordination of the Neuroptera.
Wing position at rest
The way in which insects keep their wings at rest is usually fixed for each species and includes many possibilities. However, it has only a very limited systematic value (see Fig. 1.2). Since the recent Palaeoptera cannot fold their wings backwards, the large dragonflies rest with their wings spread apart, the small dragonflies and the mayflies with their wings lying next to each other. But we also find this rest position in the day butterflies belonging to the Neoptera. The cicadas rest with wings laid together like a roof, which distinguishes them from the closely related bedbugs. The caddis flies can also be distinguished from the very similar looking small butterflies by their roof-shaped wings. With the butterfly flies belonging to the Diptera, there are also species that rest with roof-shaped wings.
Stoneflies roll their wings lengthways around the abdomen when at rest. We find something similar in some beetles that do not fold their hind wings. This is considered a primitive feature.
Occasionally keeping the wings at rest can be helpful in distinguishing between two species, e.g. B. in the butterflies.
ontogenesis
In the area of overlap between the questions on ontogeny and the insect wing, the aim is to research the point in time from which it is determined from which area of the young creature the wing arises during normal development and to what extent this area can still be reprogrammed or reprogrammed in the event of manipulated developments.
| Fig. 4.5: Discoloration of the wings after hatching in the peat damsel |
||
|---|---|---|

|

|

|
| After moulting, the wing is still widely flowed through by hemolymph , which is evident from the yellow color. Gradually , the flow of the hemolymph is restricted to lagoons, which is why the color fades. Finally, the two skin layers stick together over a large area to form a clear, transparent membrane. The veins now emerge pigmented. |
||
The so-called homeotic genes , which directly or indirectly control development, are not only very similar in all insects, but in the entire animal kingdom. In insects, during egg formation and laying by the mother, a few typical proteins or RNAs of this gene group are localized at the front and rear ends of the egg as well as dorsally. The latter cause the production of corresponding proteins during the first cell nucleus divisions. The proteins form concentration gradients through diffusion within the fertilized egg, which in their interaction as transcription factors trigger a cascade of gene expression patterns that ultimately lead to a self-regulation of the increasing differentiation during further development. At least in Drosophila it is known that the future breast section and thus the wings arise in the overlap area of the bicoid protein that diffuses from the front end already fixed on the egg and the nanos protein diffusing from the rear end.
The insect groups differ considerably in terms of the abundance of yolks in the eggs , migration of the cell nuclei after the first cell nucleus divisions , shape and movements of the germ line , the presence of the embryonic envelope , etc. Here only the basic processes of development are described and only the most important differences in connection with entered the wings. Insect eggs are rich in yolks, the proportion of yolks in exopterygotes is on average higher than in endopterygotes. After the first cell nucleus divisions, the cell nuclei arrange themselves near the surface of the egg and cell walls form starting from the outside. This first stage of development ends with the egg being surrounded by a single layer of cells. The germ line from which the embryo develops is now visible on it. While the germinal strip is stretching, a segmentation and the arrangement of paired extremity buds can be seen on the outside. They later develop into the mouthparts , legs and, in some groups, abdominal extremities; but they can also be regressed or remain fixed in the embryonic stage. In the following developmental steps the embryonic envelopes form and the germinal strip grows around the yolk mass and closes on the back. The organ systems develop and the extremities take on their larval shape. The ectoderm transforms into the epidermis, which secretes larval cuticles shortly before hatching. Haemolymph is pumped from the abdomen into the chest and head, causing them to swell. The egg shells tear and the first instar larvae hatch. Wing buds are morphologically not recognizable at this stage. In terms of further development, endo- and ectopterygotes differ significantly.
In the exopterygotes (e.g. grasshoppers and bedbugs) no wings can be seen in the first nymph stages (larval stages). Early on, however, a tiny fold of the skin can be found in the upper lateral area of the second and third breast segments, from which the wing will develop. With each moult, this fold becomes disproportionately larger to the body and more like a wing (Fig. 4.2). With the exception of the mayflies, the wings only become functional after the last moult. It must be emphasized that this development is not continuous in a threefold sense. Once, like body growth, it takes place in spurts only immediately after the moult , after the cuticle has hardened there is no longer any increase in size until the next moult. On the other hand, the development in the first moults is insignificant and appears shifted towards the last (s) of the usually four to five moults. After all, the development is not linear, but in a detour the wing initially develops in the direction of a rigid outgrowth of the notum directed backwards (Fig. 4.1, Fig. 4.2), which allows interesting speculations about the evolution already discussed. Before the last moult, the wings develop within the wing bud as a multiply folded protuberance with the structure described above, the broad base of the wing scales differentiates itself into the narrow wing joint. After the last moult, the wings stretch by pumping in hemolymph and maintain their functionality after hardening (Fig. 4.5).
The endopterygotes (beetles, flies ...) have larvae that are clearly different from the adult insect and do not show any traces of wings. Only in the pupal stage, which is considered by some to be the last larval stage in which there is no food intake, the future wings are already recognizable as a protuberance (open pupa , Fig. 4.3) or only indicated in their delineation (closed pupa, Fig. 4.4). This sudden occurrence is due to the fact that the tissue areas that were already embryonic for the wings were hormonally hindered in their development during the larval stages. Now this suppressed development is being made up for more quickly. In the range of possibilities we consider the two extremes. In species with great similarity between larva and adult, the wing formation is similar to that of the ectopterygotes, only that the wing systems are invisible from the outside inside the body and are only formed in the last larval stage (e.g. in the beaked flies). In species whose larvae are not similar to the imago, such as B. the flies, the wing formation takes place from so-called. Imaginal disks as part of a fundamental reconstruction of the body. A cavity ( peripodial cavity ) is created from an area already defined in the embryonic stage . The wing bud protrudes from the thickened bottom of this cavity into the cavity. It is initially finger-shaped and its interior is completely pervaded by hemolymph. Then it flattens out and the haemolympohe is confined to lagoons into which trachea and nerves penetrate. Finally, the top and bottom of the wing buds merge over a large area, the hemolymph is limited to canals, the future wing veins. As they develop, the wings leave the peripodial cavity through cell displacement. At the end of the pupal stage, the not yet stretched and hardened, but already fully developed wings lie against the rest of the, likewise redesigned, body and are only unfolded after they have hatched from the pupae. In the functional wing, the double layer of epidermis that was deposited by the wing membrane has died and shrunk so that the two cuticle layers lie directly on top of one another away from the veins.
Assuming that the ontogeny of the wings repeats the evolution of the wings in a modified form, one expects information from the ontology that enables conclusions to be drawn about the evolution. It turns out, however, that here too the knowledge that the comparative developmental genetics dealing with this complex of questions has so far is too incomplete and insufficiently differentiated for conclusive conclusions. In the fruit fly, for example, the embryonic areas, from which on the one hand wings and on the other hand legs arise, are identical at the earliest possible point in time for identification, which was taken as evidence of the epicoxal theory . In the meal beetle , which is also holometabolic , they are next to each other, but differ in their gene expression. It is to be expected, however, that the evolution of insects will become more understandable from an ontogenetic point of view as the amount of data on the development of the wings increases.
Duties of the wing
The functions of the wings can be classified according to natural behavior patterns such as locomotion, courtship, territorial behavior, etc. or according to movement-related aspects. The second option results in less overlap and makes it easier to mention duplicate functions.
Insect flight
Wing profile
The main task of the wings in most insects is to fly. Depending on the size, weight and type of flight of the insect, there are different requirements for wing construction.
Due to the small size of the insects, their wings have to fulfill their function with a small Reynolds number (defined here as the speed of the inflow divided by the depth of the wing profile and the viscosity of the air). They do not have the thickened and pointed aerodynamic profile known from bird wings and aircraft wings . Wings of insects show a flat shape, which is better suited for the Reynolds numbers occurring between and . In some tiny insects that live with a single-digit Reynolds number, the flight surface is replaced by fringes .
Types of flight
The flat construction is not only justified in terms of buoyancy. For example, a wing for a vertical climb, such as that of the cabbage schnake , must neither be arched nor have a profile. Otherwise forces would arise in the forward or backward direction. The flat wing shape is also advantageous for straight flight , since, unlike the curved wings of airplanes and birds, it is possible for the wings to flow on both sides. This advantage is used, for example, by insects of the genus Phormia , which use torsional vibrations to fly, for which the meaning of the wing sides is reversed several thousand times per second. For some dragonflies a top speed of 54 km / h is given. The very high acceleration is remarkable.
Insects that stay in place in so-called hovering flight , such as dragonflies and hover flies , make use of the elasticity of the wings. It is assumed that the animals can passively or even actively control the curvature of the wings through the type and frequency of flapping. However, this has so far only been proven for the migratory locust . Dragonflies and hoverflies (Fig. 0.2) are even capable of flying backwards.
Another type of flight is the so-called gliding flight, which, however, makes certain demands on the wing size of the insects. Longer gliding flights are therefore only reserved for larger butterfly and dragonfly species . Small butterflies can glide for longer periods of time, but for this they rely on special wind conditions. The American migratory butterfly monarch covers an average of 1,600 km from South America to North America; in individual cases, a flight distance of 2,800 km was measured.
Benefits of Flying
From a human perspective, the main goal of flying is to move quickly. From a biological point of view, flying primarily enables the development of new habitats. But this does not only mean that z. B. a river can be crossed and the local food sources can be developed or the flowers on a high tree can be reached. As a result of the mobility, it is now possible to change location or hike more quickly. In this way, the blossoms of different trees can be visited in quick succession or an adaptation to periodic changes in the environment is possible. It is just as important that various vital behavioral complexes could now be spatially separated, e.g. B. Foraging on the ground, reproducing in the air, and laying eggs in a tree.
Furthermore, flying makes it possible to make better use of food sources. It is not just about "finding" z. B. a flowering plant, but also about "surprising" a possible prey. At the same time, there is a need for the potential prey to escape the attack by fleeing. Not only were the shape of the wings optimized with regard to possible flight maneuvers, better eyesight was also necessary, and an antidote to the bats' tracking system was developed for night butterflies . With the flying insect, the two-dimensional areas become three-dimensional areas with all the consequences for area marking and defense.
Overall, the reason for the insects' rapid radiation is to be found in the possibility of flight .
Protective function

1. Mechanical protection: folding the wings backwards has two consequences. The advantage is that the insect takes up less space, which means that new sources of food can be developed and there are opportunities to escape and hide. However, when an insect squeezes into cracks, the risk of damaging the wings increases. It is therefore advantageous if the front wings, which come to rest on top when folding, are insensitive to mechanical damage. In some insect orders, the forewings have taken on the task of protecting not only the hind wings, but also the less chitinized and vulnerable abdomen. This protection can be achieved in a number of ways. A simple possibility results from the shape of how the wings rest against the body in the rest position and thus z. B. facilitate the sliding of parts of plants when the insect crawls through the grass. An impressive example is the cockroach, whose body contour is ideal when the wings are laid on, in order to squeeze into cracks (Fig. 6.2). Another possibility is to fold the sensitive trailing edge of the wing down so that a more robust vein forms the boundary of the wing surface when at rest (Fig. 1.3). The most effective possibility is a stronger sclerotisation (hardening) of the fore wing, which is then called the cover wing . However, this causes a higher weight, which affects the flight ability. Therefore, a compromise must be found that best suits the species' way of life. In species with poor or no flight ability, the forewings can be heavily sclerotized and fused together. If the forewings are completely or partially clearly thickened and pigmented, but still show clear veining, then they are called tegmina (singular tegmen). Are the top wing in size and shape adapted to the body outline of the abdomen and can not detect veins more, then they are called elytra (singular Elytron, Fig. 0.3). If only a part of the fore wings is more strongly developed, the term hemielytrene (Fig. 3.1) is used; if the fore wings are only moderately more robust than the hind wings, the term pseudoelytres is used.
Protection can consist in the fact that there are no injuries when the insects squeeze their way through the leaves or grass, but it can also mean that the insect becomes less attractive to a potential predator or even disappears from an insect eater's menu (Fig 6.3).
2. Camouflage must be mentioned as a further form of protection, which can be significantly improved by the color and shape of the wing covers (Fig. 6.4).
Signal function

With the strong radiation of the insects a wide range of possibilities arose to transmit more or less special messages with the wings to individuals of your own kind or other kinds. In the following, they are divided into wing drawing, wing posture and wing movement.
Coloring and color distribution
The coloring of the wings, like that of the whole insect, can either be based on pigmentation or so-called structural colors appear . Structural colors emerge especially in thin layers, as they are often found on wings or especially on the scales of butterflies (Fig. 6.5).
Color and color distribution, the so-called drawing , are subject to individual fluctuations, but are essentially species-specific. Many insects recognize potential sexual partners or competing conspecifics by the drawing of the wings. They serve as a key stimulus to trigger corresponding chains of behavior. In this way, the intensity of a drawing can become a selection advantage. With the drawing of the wing covers, some beetles imitate the drawing of the abdomen of wasps ( mimicry ).
Wing posture and movement
The wing position often signals the willingness to mate. Usually the female assumes the mating position by holding the wings in such a way that this enables mating in the first place. This is particularly easy to see in butterflies. The female Drohsophila also shows its willingness to mate by folding away the wings and exposing the genital organs. However, there are also known threats that precede an attack.
The wing movement is both more clearly visible and more plastic than the wing posture. It often occurs in connection with courtship. The waving of the sexual partner, the imposing when staking out a territory both towards the same-sex competitor and the potential mating partner and threatening are known.
The display of frightening colors by suddenly opening the wings can be found in many butterflies as well as some grasshoppers and praying mantises.
The wing movement for sound generation by means of stridulation is particularly interesting . A so-called shrill edge is moved along a shrill strip. The wings can be carriers of a shrill ledge or shrill edge or as a sound body to amplify the sounds. The species-specific chants of many insects serve to demarcate their territory, attract potential sexual partners and keep potential competitors away. They are particularly widespread in grasshoppers and crickets (Fig. 6.6 Cricket with sound example), but also occur, for example, in beetles.
More functions
With a large number of insect groups it is common for the animals to rest their wings perpendicular to the incident sun rays in order to be able to absorb as much heat as possible. This increases their operating temperature, which is an advantage for cold-blooded animals at lower temperatures. If the body is warmed up enough, this position is no longer or less often assumed. The wings are used for heat regulation.
The wings are used by bees as fans to increase the air movement when the temperature in the hive rises above 35 ° C and wax honeycombs threaten to melt. They fan moist air into the beehive, which cools down by the evaporative cold. The distribution of sexual attractants in the female silk moth has also been known for a long time . During the millennia of breeding, these butterflies lost their ability to fly. The fragrance is spread by fan movements of the reduced wings. It excites the males, who start moving towards the source of the scent. Some specialized wing scales, which then usually lie next to each other in fields and are provided with tufts of hair, so-called scented scales ( androconia ), produce odorous substances and send them out through pores. In the course of advertising for the Drosophila fruit fly , the male's rapid, whirring wing movements cause species-specific tones ("mating chants") that the female (with the antennae) hears. The wing tone of female mosquitoes (Culicidae) also serves as a signal to attract males.
Some species of oil beetle have a dome of air under their fused forewings that is believed to provide protection against tropical heat and dehydration.
It was only recently discovered that bees, during the waggle dance , transmit information about the distance to the food source through sounds they generate using wing vibration.
In aquatic insects, the wings sometimes take on tasks related to life in the water. In swimming beetles, an air bell can form under the wings into which oxygen is pumped directly from the air or into which oxygen dissolved in the water can diffuse. At the same time, the stored air influences the lift. This can be used to ensure that the movement of the insect does not have to counteract sinking or rising. In the case of the butterfly species Hydrocampa nymphaea , the air in the shell of the pupa anchored under water is held under the wings of the hatching butterflies, so that after hatching it is driven to the surface of the water like a cork. The parasitic hymenoptera of the genera Polynema , Hydrophylax , Limnodytes , Caraphractus and Aprostocetus use their wings as oars when swimming underwater.
Web links
- The rapid evolution of the insect's wings
- Image of Deltitschala bitterfeldensis
- World maps with location distribution
- Possible relationships within the Palaeooptera or Palaeopteroidea
- Possibilities of Phylogeny of the Neoptera
- Image of fore-breast appendages (Paranota), which were interpreted as a reduced 3rd pair of wings
- Further drawings on which insects with these front breast appendages can be seen (Jarmila Kukalová: Revisional Study of the Order Palaeodictyoptera in the Upper Carboniferous Shales of Commentry , France. Part IIII; Pyche vol. 77 March, 1970 No. I) ( Memento from 27. September 2007 in the Internet Archive ) (PDF; 4.5 MB)
- Photos of flying insects
Individual evidence
- ^ F. Müller: Contributions to the knowledge of termites. In: Jena. Z. Naturw. 7, 1873, pp. 333-358, 451-463.
- ↑ Grzimek's Animal Life Encyclopedia. Vol. 3, Thomson Gale, 2003, ISBN 0-7876-5779-4 .
- ↑ a b c d e f g h i j k l Cedric Gillott: Entomology . Springer Verlag, 2005, ISBN 1-4020-3182-3 in.
- ↑ Raymand C. Moore, among others: Invertebrate Fossils. MacGraw-Hill, 1952.
- ^ A b Joel G. Kingsolver, MAR Koehl: Selective Factors in the Evolution of Insect Wings . In: Annual Revue of Entomology . tape 39 , 1994, pp. 425-451 .
- ^ A b E. L. Jockusch, KA Ober: Hypothesis Testing in Evolutionary Developmental Biology: A Case Study from Insect Wings . In: Journal of Heredity . tape 95 , no. 5 , 2004, p. 382-396 .
- ↑ a b c Günter Bechly: Ur-Geziefer - The fascinating evolution of insects . In: Stuttgart contributions to natural history series c - knowledge for everyone . tape 49 , 2001, p. 35-38 .
- ^ N. Niwa et al .: Evolutionary origin of the insect wing via integration of two developmental modules. In: Evolution & Development. 12 (2), 2010, pp. 168-176.
- ↑ The rapid evolution of the insect wing. on: ag-evolutionsbiologie.net
- ^ Arnold H. Staniczek, Günter Bechly, Roman J. Godunko: Coxoplectoptera, a new fossil order of Palaeoptera (Arthropoda: Insecta), with comments on the phylogeny of the stem group of mayflies (Ephemeroptera). In: Insect Systematics & Evolution. 42 (2), Brill, Leiden 2011, pp. 101-138. ISSN 1399-560X ( full text as PDF; 44 MB ( memento of April 13, 2014 in the Internet Archive )).
- ^ A. P Rasnitsyn, DLJ Quicke: History of insects. Kluwer Academic Publishers, 1980. (Presentation with excerpts on: palaeoentomolog.ru )
- ↑ a b c Klaus-Dieter Klaß: The phylogeny of the Dictyoptera. Cuvillier Verlag, Göttingen 1995, ISBN 3-89588-363-8 .
- ↑ a b c d e Fabian Haas : Geometry, mechanics and evolution of wing folding in the Coleoptera. PhD thesis. University of Jena, 1998.
- ^ Robin J. Wootton: The Design of Insect Wings . In: Spectrum of Science . January 1991, p. 58-65 .
- ^ A b Fabian Haas: Evidence from Folding and Functional Lines of Wings on Inter-ordinal Relationships in Pterygota . In: Arthropod Systematics & Phylogeny . tape 64 , no. 2 , 2006, p. 149-158 .
- ↑ Paul Brohmer (Ed.): Fauna von Deutschland. Quelle and Meyer, Heidelberg 1964.
- ↑ a b G. Seifert: Entomological internship. Georg Thieme Verlag, Stuttgart / New York 1995, ISBN 3-13-455003-2 .
- ↑ dtv atlas on biology. Deutscher Taschenbuchverlag, Munich 1971, ISBN 3-423-03011-9 .
- ↑ a b c d Michael Chinery: Parey's book of insects. Parey, Hamburg / Berlin 1993, ISBN 3-490-23118-X .
- ↑ a b Large Lexicon of the Animal World. Lingen Verlag, Cologne.
- ^ BN Danforth, CD Michener: Wing folding in the Hymenoptera. In: Annals of the Entomological Society of America. 81 (2), 1988, pp. 342-349.
- ↑ a b c H. Freude, KW Harde, GA Lohse: Die Käfer Mitteleuropas. Volume 9, Spektrum Akademischer Verlag in Elsevier, 1966, ISBN 3-8274-0683-8 .
- ↑ Gerald Moritz: Thrips. (= Die Neue Brehm-Bücherei. Volume 663). Westarp Sciences, Hohenwarsleben 2006, ISBN 3-89432-891-6 .
- ^ Jill Silsby: Dragonflies of the World. The National History Museum, 2001, ISBN 0-565-09165-4 , p. 180.
- ↑ Heiko Bellmann : The new Kosmos butterfly guide. Butterflies, caterpillars and forage plants. Franckh-Kosmos, Stuttgart 2003, ISBN 3-440-09330-1 .
- ^ Lionel G. Higgins, Norman D. Rilley: The butterflies of Europe and Northwest Africa. (A Field Guide to the Butterflies of Britain and Europe), Paul Parey Verlag, 1971, ISBN 3-490-02418-4 .
- ↑ W. McGinnis, M. Kuziora: Control genes for the body plan . In: Spectrum of Science . April 1994, p. 38 .
- ↑ Christiane Nüsslein-Volhard: Gradients as organizers of embryonic development . In: Spectrum of Science . October 1996, p. 38 .
- ^ W. Nachtigall: Insect flight. Springer-Verlag, Berlin / Heidelberg / New York 2003.
- ↑ Peter Detzel: The locusts of Baden-Württemberg. Verlag Eugen Ulmer, Stuttgart 1998, ISBN 3-8001-3507-8 .
- ↑ z. E.g .: Therese Ann Markow, Patrick M. O'Grady: Evolutionary Genetics of Reproductive Behavior in Drosophila: Connecting the Dots. In: Annual Review of Genetics . 39, 2005, pp. 263-291. doi: 10.1146 / annurev.genet.39.073003.112454
- ↑ Bernhard Klausnitzer: Wonderful world of the beetles . Herder Verlag, Freiburg 1982, ISBN 3-451-19630-1 .
- ↑ Wolfgang Engelhardt: What lives in pools, brooks and ponds . Kosmos, Franckh'sche Verlagshandlung, Stuttgart 1955.